eISSN: 2574-9927


Mini Review Volume 1 Issue 3
Department of Applied Chemistry, Babasaheb Bhimrao Ambedkar University, India
Correspondence: Azad Kumar, Department of Applied Chemistry, Babasaheb Bhimrao Ambedkar University, India
Received: August 10, 2017 | Published: November 8, 2017
Citation: Kumar A, Pandey G. A review on the factors affecting the photocatalytic degradation of hazardous materials. Material Sci & Eng Int J. 2017;1(3):106-114. DOI: 10.15406/mseij.2017.01.00018
The oxidation rates and efficiency of the photocatalytic system are highly dependent on a number of operational parameters that govern the photodegradation of the organic molecule. Several study have been reported the significance of operational parameter. The photodegradation depends on the some basic parameters which are concentration of substrate, amount of photocatalyst, pH of the solution, temperature of reaction medium, time of irradiation of light, the intensity of light, surface area of photocatalyst, dissolve oxygen in the reaction medium, nature of the photocatalyst, nature of the substrate, doping of metal ions and non metal and structure of photocatalyst and substrate. The photodegradation of organic compound have been studied by the several scientists and conclude the optimum conditions for the photodegradation of organic compound.
The photodegradation of organic compounds was found maximum at low concentration of organic substrate with optimum amount of photocatalyst. The pH of the solution is also affect the photodegradation of organic substrate. The Titania show the maximum adsorption at low pH hence the photodegradation also found maximum at low pH. The surface area is the crucial factor for the photodegradation of organic substrate. If we are increasing the surface area of photocatalyst, the photodegradation of organic substrate increase. This is because that the number of active site increased with increasing the surface area. The amount of photocatalyst is the primary requirement of any photocatalysis process. The amount of photocatalyst should be optimum, if we take the high amount of photocatalyst the photodegradation is decreased, if we take the low amount of photocatalyst the photodegradation also decreased. The doping of metal ions and non-metal ions affect the photodegradation of organic substrate. Therefore, we have to use the metal ions which increased the positive charge on the surface of photocatalyst. The temperature and irradiation of light also affect the photodegradation of organic substrate. For the maximum photodegradation, the photocatalytic reaction should perform at room temperature not greater than 80°C. The light of irradiation should be used which exit the electron easily from valence band to conduction band or equal to band gap energy.
Keywords: photodegradation, photocatalyst, surface area, nanocomposites, titania, metal doped titania
The industrial waste water containing the various dyes is responsible for water pollution, due to their carcinogenic behaviour. Lots of investigations reported that 10-12% of dyes are used every year in textile industries such as Rose Bengal, Victoria blue, Thymol blue, Caramine, Indigo Red, Red 120, Rhodamine B, Methylene Blue, Eriochrome Black-T (EBT) 1-4 of which are major portion (20%) lost during synthesis and processing operations, which enter into water through effluents. The wastewater released from industries contains highly hazardous and coloured pigments which causes serious ill effect on aquatic life and also human beings.5-7
In the past, several physical techniques like photo degradation, coagulation, flocculation, reverse osmosis, adsorption on the activated carbon, ion exchange method ultra-filtration and chemical methods like photosensitized oxidation, adsorption,15 have been used to reduce the toxic dye effluents from waste water9-12 though methods are fairly effective in removing pollutants. However the main drawback of these techniques is formation of secondary waste product which cannot be treated again and dumped as such.13,14
The photo catalysis is the very advanced oxidation processes (AOP) which is used for the photo degradation of toxic compounds. It is also used for the purification of water.15-17 Basically, the photo catalysis is divided into two categories (1) heterogeneous catalysis and (2) homogenous catalysis. Heterogeneous catalysis has been successfully employed for the degradation of various families of hazardous materials. The photodegradation has lot of advantages over traditional wastewater treatment techniques such as chemical oxidation,18 activated carbon adsorption,19 biological treatment,20 etc. Activated carbon adsorption method involves the phase transfer of pollutants without decomposition and thus creates another pollution problem. Chemical oxidation method is unable to remove all organic substances and it is suitable for the removal of pollutants at high concentrations. The biological treatments are very slow, dispose large amount of sludge and required strict control of proper pH and temperature.21 In this regard photo catalytic processes have advantages for the removal of pollutants even at low concentration for industrial waste water.22 Moreover in photo oxidation, complete oxidation of organic pollutants take place within few hours, even at ppb level, without formation of secondary hazardous products using highly active and cheap catalysts which can be used in specially design reactor systems.23
Titanium dioxide is a widely accepted photocatalyst due to its high oxidation efficiency, non-toxicity, high photo stability, chemical inertness and environmental friendly nature.24,25 It is a wide band gap (
3.2eV) semiconductor and mineralizes a large range of organic pollutants such as herbicides, dyes, pesticides, phenolic compounds, tetracycline, sulfamethazine, etc under UV irradiation.26 In photocatalytic processes the photon with energy, equal to or greater than the energy gap (3.2eV) is absorbed by TiO2 particles, therefore holes (h+) are created in the valence band and electrons (e-) in the conduction band, which diffuses to the surface of TiO2 and participates in redox reactions of the adsorbed substrates.27 In brief, photo generation of radical species in TiO2/UV system can be described as follows.28,29
(1)
(2)
(3)
(4)
(5)
(6)
(7)
(8)
Where hvis the photon energy which is required to excite the semiconductor electron from the valence band (VB) to conduction band (CB).
Factors influencing the photocatalytic degradation
The oxidation rates and efficiency of the photocatalytic system are highly dependent on a number of operational parameters that govern the photodegradation of the organic molecule.30-34 Several study have been reported the significance of operational parameter.
Effect of dye concentration
The photocatalysis is depends on the adsorption of dyes on the surface of photocatalyst. In the photocatalysis process, only the amount of dye adsorbed on the surface of photocatalyst contributes and not the one in the bulk of the solution. The adsorption of dye depends on the initial concentration of dye. The initial concentration of dye in a given photocatalytic reaction is an important factor which needs to be taken into account. Generally speaking the percentage degradation decreases with increasing amount of dye concentration, while keeping a fixed amount of catalyst.35
This can be rationalized on the basis that as dye concentration increases, more organic substances are adsorbed on the surface of TiO2, whereas less number of photons are available to reach the catalyst surface and therefore less •OH are formed, thus resulting in less degradation percentage. Figure 1 is showing the photodegradation of methyl orange with different photocatalyst. The photodegradation of dyes are increase with decreasing the concentration of dyes with the photocatalyst.36
Effect of catalyst amount
Degradation of dye is affected by the amount of the photocatalyst. The photodegradation of dye increases with increasing catalyst amount, which is the feature of heterogeneous photocatalysis. The increase in catalyst amount actually increases the number of active sites on the photocatalyst surface thus causing an increase in the formation of number of •OH radicals which can take part in actual discoloration of dye solution. Beyond a certain limit of catalyst amount, the solution becomes turbid and thus blocks UV radiation for the reaction to proceed and therefore percentage degradation starts decreasing.37
Figure 2 showing the photodegradation of methyl orange dye at different amount. The percent photodegradation is increase with increasing the amount of photocatalyst.36 Figure 3 is also showing the effect of photocatalyst amount on the photodegradation of Eriochrome black T (EBT). The Photodegradation of EBT is also increase with amount of photocatalyst.38 Figure 4 showing the photodegradation of acetic acid in presence of Titania and Co, Ni modified Titania at different amount. The photodegradation of acetic acid was increased with increase of photocatalyst amount.39
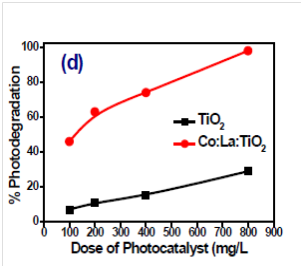
Figure 2 Effect of photocatalyst amount on photodegradation of Methyl Orange with (a) TiO2 (b) Co: La: TiO2 [36].
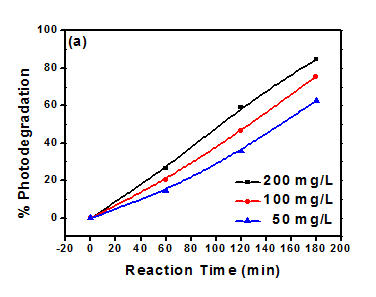
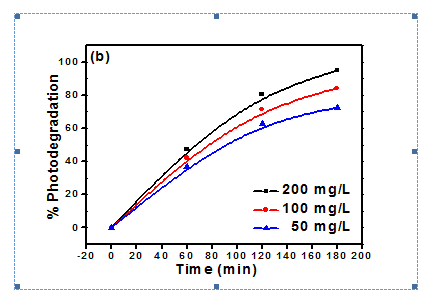
Figure 3 Effect of photocatalyst amount on Photodegradation of EBT with (a) TiO2 (b) Ni:TiO2.38
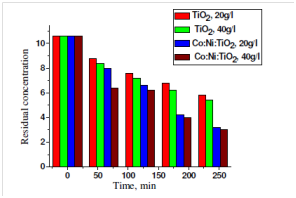
Figure 4 Effect of photocatalyst amount on Photodegradation of Acetic acid with TiO2 and Co:Ni:TiO2.39
Effect of pH
The Photodegradation of dyes are affected by the pH of the solution. The variation of solution pH changes the surface charge of TiO2 particles and shifts the potentials of catalytic reactions. As a result, the adsorption of dye on the surface is altered thereby causing a change in the reaction rate. Under acidic or alkaline condition the surface of Titania can be protonated or deprotonated respectively according to the following reactions:40
(9)
(10)
Thus titania surface will remain positively charged in acidic medium and negatively charged in alkaline medium. Titanium dioxide is reported to have higher oxidizing activity at lower pH, but excess H+ can decrease reaction rate. TiO2 behaves as a strong Lewis acid due to the surface positive charge. In other words, the anionic dye acts as a strong Lewis base and can easily adsorb on the positively charged catalyst surface. This favours the adsorption of the dye under acidic conditions, while in the alkaline conditions this complexation process is not favoured presumably because of competitive adsorption by hydroxyl groups and the dye molecule in addition to the Columbic repulsion due to the negatively charged catalyst with the dye molecule.41 The extent of dye adsorption depends on the initial dye concentration, nature of the dye, surface area of photocatalyst and pH of the solution. The pH determines the surface charge of the photocatalyst. Adsorption of the dye is minimum when the pH of the solution is at the isoelectric point (point of zero charge.42 The surface of the photocatalyst is positively charged below isoelectric point and carries a negative charge above it. Figures 5-7 showing the effect of pH on the photodegradation of methyl red,43 EBT38 and Oxalic Acid.44 The Photodegradation of Methyl Red is found maximum at 5pH because the methyl Red is the cationic dye which adsorb easily on the surface of Titania (Figure 5). The photodegradation of EBT is found maximum at 2pH because it is anionic dye which adsorb maximum at 2pH. Hence maximum photo-degradation occurs at 2pH (Figure 6). Figure 7 showing the effect of pH on photodegradation of oxalic acid at two temperatures with Cu-TiO2. The photodegradation of Oxalic acid was found maximum at 4pH. This is because that in acetic medium the adsorption of Oxalic acid is found maximum. Therefore, the photodegradation of oxalic acid was found maximum at 4pH.
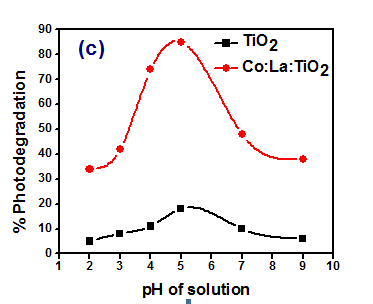
Figure 5 Effect of pH of solution on photodegradation of Methyl red with TiO2 and Co:La:TiO2.43


Figure 6 Effect of pH on Photodegradation of EBT at various concentrations (a) TiO2 (b) Ni:TiO2.38

Figure 7a The effect of pH on the photodegradation of Oxalic Acid at different temperature with Cu-TiO2.44
Size and structure of the photocatalyst
Surface morphology such as particle size and agglomerate size, is an important factor to be considered in photocatalytic degradation process because there is a direct relationship between organic compounds and surface coverage of the photocatalyst.45 The number of photon striking the photocatalyst controls the rate of reaction which signifies that the reaction takes place only in the absorbed phase of the photocatalyst.46 A number of different forms of TiO2 have been synthesized to achieve the desired characteristics of the photocatalyst.47 Figure 8 showing the photodegradation of methyl red dyes with Titania and Cu-TiO2 nanocomposites. The maximum photo-degradation is found in case of Cu modified Titania and whereas in case of Titania less photodegradation occurs.43 This is because that the surface of Titania different from Cu modified Titania. The Cu modified Titania has rough and zigzag surface which is help in the adsorption of organic molecule on the catalyst surface. So that Cu modified Titania shows good photocatalytic activity than pure Titania.
Figure 9 shows the effect of different structures on the photodegradation of EBT. The nanoparticle of Titania which is P25-TiO2 is the superior photocatalyst than the Titania nanotubes and nanorods. This is because that the nanoparticles have higher surface area than the nanotubes and nanorods. The nanoparticles show the 86-94% degradation of EBT.

Figure 9 Photodegradation of Methyl red at initial concentration 50 ppm (a) TiO2 at 30 °C (b) TiO2 at 40 °C (c) Co:La:TiO2 at 30 °C (d) Co:La:TiO2 at 40 °C [43].
Surface area
Surface morphology of TiO2 is a crucial factor in its use as photocatalyst, as all the chemical events take place at the surface, its enlargement has been attempted, usually by using very fine particles, either suspended in solvents or made into a porous film. Nanostructured materials with the crystallite/grain size below 20nm are of great research interest mainly due to the fact that their physical properties may be markedly different from the bulk counter parts. This has also opened up avenues for their applications as photocatalyst in numerous areas.48 Figure 10 shows the effect of surface area on photodegradation of rose Bengal dye in presence of PAni/Graphene nanocomposites. The surface area of photocatalyst increases the photodegradation of rose Bengal also increase. This is because that the high surface area contains the greater no of active site than the low surface area materials.
Reaction temperature
An increase in reaction temperature generally results in increased photocatalytic activity however reaction temperature >80 °C promotes the recombination of charge carriers and disfavour the adsorption of organic compounds on the Titania surface.49 A reaction temperature below 80 °C favours the adsorption whereas further reduction of reaction temperature to 0 °C results in an increase in the apparent activation energy.50 Therefore temperature range between 20-80 °C has been regard as the desired temperature for effective photo mineralization of organic content.51
Figures 11 & 12 showing the effect of temperature on the photo -degradation of methyl red and methyl orange in presence of TiO2 and La and Co modified Titania. At the high temperature the photodegradation was found maximum whereas at low temperature the photodegradation of dyes was found low efficiency of photodegradation. This is due the interaction of dyes molecule increase with the photocatalyst surface. Hence the adsorption increases which favour the photodegradation of dyes.36,43
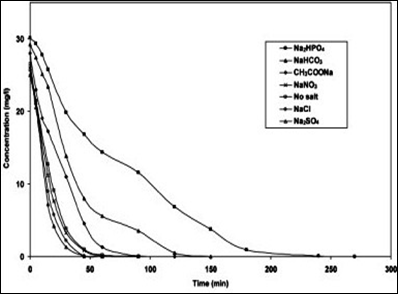
Figure 11 Effect of anions on the photodegradation of RY 145 (Co = 30mg/l).67
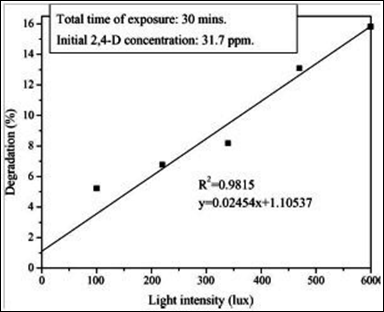
Figure 12 Effect of light intensity on 2,4-D photodegradation.70
Concentration and nature of pollutants
The rate of photocatalytic degradation of certain pollutant depends on its nature, concentration and other existing compounds in water matrix. A number of studies have reported the dependency of the TiO2 reaction rate on the concentration of contaminants in water.52 High concentration of pollutants in water saturates the TiO2 surface and hence reduces the photonic efficiency and deactivation of the photocatalyst.53 In addition to the concentration of pollutants, the chemical structure of the target compound also influences the degradation performance of the photocatalytic reactor. For example, 4-chlorophenol requires prolonged irradiation time due to its transformation to intermediates compared with oxalic acid that transforms directly to carbon dioxide and water, i.e., complete mineralization.54 Furthermore if the nature of the target water contaminants is such that they adhere effectively to the photocatalyst surface the process would be more effective in removing such compounds from the solution. The photocatalytic degradation of aromatics is highly dependent on the substituent group.55 The organic substrates with electron withdrawing nature (benzoic acid, nitrobenzene) strongly adhere to the photocatalyst and therefore are more susceptible to direct oxidation compared with the electron donating groups.56
Inorganic ions
Various inorganic ions such as magnesium, iron, zinc, copper, bicarbonate, phosphate, nitrate, sulphate and chloride present in wastewater can affect the photocatalytic degradation rate of the organic pollutants because they can be adsorbed onto the surface of TiO2.57 Photocatalytic deactivation has been reported whether photocatalyst is used in slurry or fixed-bed configuration which is related to the strong inhibition from the inorganic ions on the surface of the TiO2.58 A number of studies have been conducted on the effect of inorganic ions (anions and cations) on TiO2 photocatalytic degradation.59 Some of the cations such as copper, iron and phosphate have been reported to decrease the photodegradation efficiency if they are present at certain concentrations whereas calcium, magnesium and zinc have little effect on the photodegradation of organic compounds which is associated to the fact that these cations have are at their maximum oxidation states that results in their inability to have any inhibitory effect on the degradation process.60
The inorganic anions such as nitrate, chlorides, carbonates and sulphates are also known to inhibit the surface activity of the photocatalyst. The presence of salts diminishes the colloidal stability, increases mass transfer and reduces the surface contact between the pollutant and the photocatalyst.61 Other than fouling of the TiO2 surface certain anions such as chlorides, carbonates, phosphate and sulphates also scavenge both the hole and the hydroxyl radicals.62 The mechanism of whole and radical scavenging by chloride has been proposed by Matthews and McEnvoy63 as follows.
![]() (11)
(11)
![]() (12)
(12)
The inhibitory effect of chloride ions occurs through preferential adsorption displacement mechanism which results in reducing the number of OH- available on the photocatalyst surface.64 The fouling of photocatalytic surface can be reduced by pre-treatment of water such as with ion exchange resins which have been reported to reduce the fouling and so the cost of treatment.65 Similarly the fouling induced by sulphates and phosphates has been reported to be displaced by NaOH, KOH and NaHCO3.66 However, most of studies conducted on the effect of inorganic ions are based on the model compounds and therefore do not necessarily represent their effect in real water matrix where several ions exist. More work concentrating on the effect of complex mixtures of inorganic ions is thus required. Figure 13 showing the effect of different ions on the photodegradation of RY 145 dye at initial concentration of 30 mg/L.
Both light intensity and time of irradiation affect the dye degradation.68 It has been shown that at low light intensities (0-20mW/cm2), the rate would increase linearly with increasing light intensity (first order), whereas at intermediate light intensities (25mW/cm2) the rate would depend on the square root of the light intensity.69 At high light intensities the rate is independent of light intensity, because at low light intensity reactions involving electron–hole formation are predominant and electron–hole recombination is negligible. On the other hand, when light intensity is increased, the electron–hole pair separation competes with recombination, thereby causing lower effect on the reaction rate.
Figure 14 shows the effect of light intensity on the photodegradation of 2,4-D. The photodegradation of 2,4-D is increased with increasing the light intensity from 100 to 600 Lux. In many literature studies, it has been shown that the dye decolorization initially increases as the light intensity is increased.70
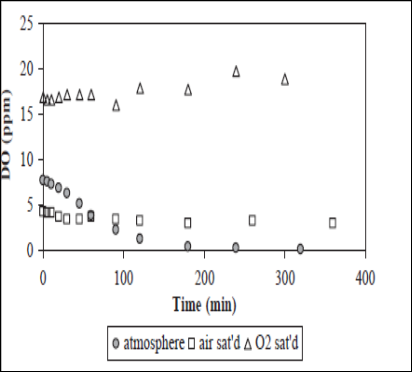
Figure 14 Effect of dissolved oxygen concentration on photocatalytic degradation, initial dye concentration=250ppm, TiO2 loading=1g/L, flow rate=1L/min.74
The reaction rate decreases with irradiation time as it follows the pseudo first-order kinetics and additionally a competition for degradation may occur between the reactant and the intermediate products. The slow kinetics of dye degradation after certain time limit is mainly attributed to the difficulty in the reaction of short chain aliphatics with •OH radicals, and the short lifetime of photocatalyst because of active sites deactivation by strong by-products deposition.71 Figure 15 shows the effect of irradiation time on the photodegradation of methyl orange with Titania and Co and La modified Titania. The photodegradation of methyl orange was found highest at 3 hour irradiation of visible light on the photocatalytic reaction. Hence this is confirm that the photodegradation increase with increase of time of irradiation on the photocatalytic reaction.

Figure 15 Comparison of decolouration course of Acid Orange 7 solutions (C0 0:1mM) in the presence of TiO2 (2.5g/l), FeCl3 (1.0mM) and TiO2 (2g/l)/FeCl3 (1.0mM).83
Dissolved oxygen
Oxygen dissolved in solution is commonly employed as an electron acceptor in photocatalysis reaction to assure sufficient electron scavengers present to trap the excited conduction band electron from recombination.72 The oxygen does not affect the adsorption on the TiO2 catalyst surface as the reduction reaction takes place at a different location from where oxidation occurs. Dissolved oxygen involves in the stabilization of radical intermediates, mineralization and direct photocatalytic reactions. Its presence is also known to induce the cleavage mechanism for aromatic rings in organic pollutants that are present in the water matrices.73 Figure 16 shows the Effect of dissolved oxygen concentration on photocatalytic degradation, at initial dye concentration=250ppm, TiO2 loading=1g/L, flow rate=1L/min. The photodegradation of dye increased with increasing the DO. This is due to the formation of Oxygen free radical which increases the photodegradation.
Effect of dopants on dye degradation
Heterogeneous photocatalysis involving titanium dioxide (TiO2) appears to be the most promising technology for organic dyes degradation. However one of the major problems in using TiO2 as a catalyst is the low photo-quantum efficiency which arises from the fast recombination of photogenerated electrons and holes. Moreover, TiO2 is inactive under visible light due to its wide band gap (3.03eV for rutile and 3.18 for anatase form). This inherently causes the inability to make use of the vast potential of solar photocatalysis. Various techniques have been employed to make TiO2 absorb photons of lower energy as well. These techniques include surface modification via organic materials and semiconductor coupling, band gap modification by creating oxygen vacancies and oxygen sub-stoichiometry, by nonmetals including co-doping of nonmetals and metal doping. Dopants, such as transitional metals have been added to the TiO2 catalyst to improve its response and also reduce the recombination of photogenerated electrons and photogenerated holes.74,75 The main objective of doping is to induce a bathochromic shift, i.e., a decrease of the band gap or introduction of intra-band gap states, which results in more visible light absorption. The effect of metal ion dopants on the photocatalytic activity is a complex problem. The total induced alteration of the photocatalytic activity is made up from the sum of changes which occur in the light absorption capability of the TiO2 photocatalyst, adsorption capacity of the substrate molecules at the catalyst's surface and interfacial charge transfer rate. Using suitable transitional metals as dopants improves the performances of TiO2.76,77 This results in the overlap of the conduction band due to Ti (3d) with d levels of the transition metals causing red shift of the band edge of TiO2. It can thus also allow the light absorption to be widened into the visible region to various extents, depending on the type of the dopant and its concentration. Therefore the photocatalysis on modified TiO2 can be promoted using visible light. Various transition metals, such as Ni2+, Zn2+, Cr3+ and Fe3+ can be easily incorporated into the crystal lattice of TiO2 because of their similar ionic radii (The ionic radii of Ni2+=0.72 Å, Zn2+=0.74 Å, Cr3+= 0.76 Å and Fe3+=0.69 Å are quite similar to that of host Ti4+=0.75 Å ions).78-81 The degradation of dyes is usually faster in mixed systems as compared to single systems, because the oxidation of dyes consumes photo-excited holes promptly and efficiently, thus attenuating electron–hole recombination.82 Figure 17 showing the Effect of Cl ion on the photodegradation of acid orange dye. The photodegradation increased with the impregnation of Cl ion in Titania.83
None.
The author declares no conflict of interest.

©2017 Kumar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.