MOJ
eISSN: 2475-5494


Mini Review Volume 10 Issue 4
Director & Chief of Andrology, Andrology Institute of America, USA
Correspondence: Panayiotis Michael Zavos, Director & Chief of Andrology, Andrology Institute of America and Theodora Maria Zavos, Visiting Scholar, Andrology Institute of America, Lexington, Kentucky, USA
Received: June 27, 2021 | Published: July 29, 2021
Citation: Zavos PM. Declining sperm counts: The impact of covid-19 on testicular function and sperm parameters and overall male reproductive performance. MOJ Women’s Health. 2021;10(4):85-89. DOI: 10.15406/mojwh.2021.10.00294
Male infertility is linked to some viral infections including human papillomavirus (HPV), herpes simplex viruses (HSV) and human immunodeficiency viruses (HIVs). As for acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), its effects on worldwide declines in sperm count and fertility have not been researched thoroughly. With the recent increase of viral infections due to the pandemic, the potential negative impacts that SARS-CoV-2 will have on male reproductive organs and male fertility have raised countless concerns. This review article aims to discuss the possible effects that the SARS-CoV-2 pandemic will have on an already declining male reproductive success while integrating the results of recent studies focusing on similar topics. Furthermore, this article will also mention the future implications that come with a more infertile population. Within the articles studied, it has become apparent that the SARS-CoV-2 pandemic has and will only decrease men’s sperm quality further. These findings became apparent through the study of oxidative stress established through the sperm’s production of reactive oxygen species1 and the COVID-19 virus’ ability to attack human spermatozoa produced in the testes due the expression of the ACE2 gene.2 As for the decline in male fertility prior to the SARS-CoV-2 pandemic, there are many factors to be discussed, some of which include: tobacco consumption, alcoholism, diet, electronics, and higher rates of testicular cancer.3
As documented in various studies, male infertility is linked to some viral infections including human papillomavirus (HPV), herpes simplex viruses (HSVs) and human immunodeficiency viruses (HIVs). However, when it comes to severe acute respiratory coronavirus (SARS-CoV-2), not much knowledge has been acquainted regarding its effects on male reproductive success. However, according to several sources, the new coronavirus, known as SARS-CoV-2, can enter human cells and cause tissue damage by binding its spike protein a cell membrane protein angiotensin-converting enzyme 2 (ACE2; See Figure 1).
The ACE2 gene is known to be present in several human organs in addition to the lungs where the virus seems to be most abundant; some of these human organs include the kidney, brain, and heart. Furthermore, there is also an abundance of ACE2 located in the human testes which explains its subjection to viral attacks and future decrease in male fertility post COVID-19 infection. Moreover, this ACE2 gene is also concentrated in several cells which are directly related to the male reproductive system and its overall physiology which makes the male reproductive system even more vulnerable to SARS-CoV-2. Cells present in the parenchymal testes in which ACE2 is expressed, are therefore vulnerable to COVID-19 infection and include the germ cells, supporting (Sertoli) cells, and Leydig cells. The germ cells’ expression of this ACE2 gene is most definitely detrimental to a man’s reproductive system considering SARS-CoV-2 will be present in human spermatozoa and potentially the mature spermatozoa being involved in the fertilization process.4 Also, the COVID-19 virus infecting Leydig cells will impact the cell’s receptivity to LH stimulation which signifies a decrease in the LH-to-testosterone ratio; a common mechanism present in men that have contracted HIVs, HSVs, and HPV rendering them infertile.4 This claim regarding the SARS-CoV-2 infection along with its entry into human cells using the ACE2 gene and the possible risk factors of COVID-19 (SARS‐CoV‐2) infection on fertility comes from receptor entry of the virus in the testes, a reduction/alteration in vital sex hormone ratios and SARS‐CoV‐2‐associated fever is supported in ‘Coronavirus disease-19 and fertility: viral host entry protein expression in male and female reproductive tissues published by Stanley et al.5
In a study conducted by Zavos et al.1 at the Andrology Institute of America (AIA), the quality of ejaculates and positive sperm DNA fragmentation characteristics between COVID‐19 infected males and their non-infected counterparts were studied. Furthermore, the experimental design employed had as objectives to look at those effects within the same male population, before and after those subjects were infected with COVID-19. Additionally, investigation pertaining to oxidative stress established through the sperm’s production of reactive oxygen species and/or the disruption of antioxidant defense systems in the male reproductive tract was carried out using the subject’s sperm samples. Two groups of patients of similar age characteristics that were attending the Andrology Institute of America (AIA) were then asked to give a semen sample in order to isolate and study their corresponding spermatogenic parameters. Patients in Group 1 (N=30) consisted of males that were not infected with SARS‐CoV‐2 and patients in Group 2 (N=30) consisted of individuals who were infected with SARS‐CoV‐2 and also underwent andrological evaluation at the AIA before and after being infected. All of the subjects’ sperm samples from group two were evaluated approximately 90 days post COVID-19 infection (one spermatogenic cycle). Seminal collections and evaluations were performed and assessed as previously described.6 Similarly, DNA fragmentation assessments and reactive oxygen species (ROS) measurements were performed following established protocols.1,7,8
The data obtained from the published results by Zavos et al.1 on COVID-19 and its effects on male fertility establish very clearly those effects throughout the study. Table 1 depicts the results obtained of the Covid effects on the men’s spermatogenic parameters while Table 2 highlights similarly the data collected from the sperm cell’s DNA fragmentation and reactive oxygen species. Groups 1 and 2 contained subjects of uniform age ranges to make the data as reliable and objective by removing any aging effect between the two groups studied.
|
Men (N) |
Ages (Yrs.) |
Volume (mL) |
Count/mL (x106) |
Total count (x106) |
Motility (%) |
Grade (0-4) |
Morphology (% Normal) |
|
Uninfected N-30 Time dif. in days |
41.2 ± 3.1 |
3.2 ± 0.7a 3.1 ±.0.6a |
37.5 ± 2.6a 39.6 ± 2.9a |
120 ± 3.1a 123 ± 3.6a |
47.8 ± 7.6a 45.9 ± 6.1a |
3.5 ± 0.6a 3.4 ± 0.5a |
13.1 ± 0.4a 12.7 ± 0.3a |
|
Infected N=30 Time dif. in days |
40.5 ± 4.6 110 ± 9.7 |
3.7 ± 0.6a 3.2 ± 0.8a |
34.2 ± 2.5a 19.3 ± 5.1b |
127±3.8a 62 ± 6.3b |
43.7 ± 4.2a 31.2 ± 6.1b |
3.3 ± 0.5a 2.1 ± 0.6b |
12.5 ± 0.5a 6.3 ± 1.7b |
Table 1 Represents the clinical data regarding analyzed spermatogenic parameters between Groups 1 and 2. For Group 2, both pre- and post-infection data is displayed; values were taken 110+9.7 days apart. For Group 1 subjects, there was no pre- and post-infection period, but values were taken 96.0+5.1 days apart (Means+/- SD)
a,b Means with different superscripts within columns are significantly different (P<0.05)
|
Men (N) |
Ages (Yrs.) |
DNA Fragment. (%) |
ROS RLU/sec/106 sperm |
|
Uninfected N-30 Time differ. in days |
41.2 ± 3.1 96.0 ± 5.1 |
8.3 ± 1.3a 9.1 ± 1.5a |
0.50 ± 0.01a |
|
0.60 ± 0.01a |
|||
|
Infected N=30 Time differ. in days |
40.5 ± 4.6 110 ± 9.7 |
10.3 ± 1.7a 33.7 ± 5.1b |
0.32 ± 0.01a |
|
34.32 ± 4.70b |
Table 2 Represents the clinical data concerning ROS values and DNA fragmentation between Groups 1 and 2. For Group 2, both pre- and post-infection data is displayed; values were taken 110+9.7 days apart. For Group 1 subjects, there was no pre- and post-infection period, but values were taken 96.0+5.1 days apart
a,b Means with different superscripts within columns are significantly different (P<0.05)
When analyzing the data collected regarding sperm parameters within Group 1 (non-infected with SARS-CoV-2) and during both time increments of approximately 96.0+5.1 days apart, it was clear that no significant deviations were present (P<0.05). According to the World Health Organization standards, spermatogenic parameters established from the subjects’ profile in Group 1 were and stayed within normal ranges. However, subjects in Group 2 who were infected with COVID-19 seemed to establish significant differences in the results regarding their spermatogenic parameters as compared between the pre and post infection periods. Although it was made clear that the same comparisons were carried out between the two time periods (110+9.7 days apart), in Group 2 it became abundantly clear that there was a considerable decrease in almost all of the spermatogenic parameters evaluated (P<0.05).
Data as depicted in Table 2, DNA fragmentation and ROS values have been recorded from the same study. When considering the data, it becomes evident that COVID-19 not only affected sperm parameters, but also reduced the stability of the sperm cells’ DNA. Similarly, when Group 2 patient’s sperm samples were compared before COVID-19 infection and 110 days post infection, it was noticed that the values were significantly different (P<0.05). Furthermore, in Table 2, ROS values were also displayed and show similar trends to the other two areas of sperm qualities measured. In Group 2, ROS levels appear to be highest following COVID-19 infection, where the pre-infection subjects in Group 2 displayed a mean ROS value of 0.32 versus a mean ROS value post-infection of 14.32 RLU/sec/106 sperm (P<0.05). As assumed, Group 1 subjects displayed no differences between the two testing time periods with mean values of 0.50 RLU/sec/106 sperm versus 0.60 RLU/sec/106 sperm, respectively. Overall, when considering all of the generated data regarding ROS levels, a strong positive correlation (r = 0.8147, P < 0.0001) between pre-infection and post-infection sperm samples was evident.
As mentioned in this article, male infertility is subjected over the years to a number of viral infections including human papillomavirus (HPV), herpes simplex viruses (HSV) and human immunodeficiency viruses (HIVs). As for SARS-CoV-2, there is little evidence circulating in the scientific world pertaining to its effects on male fertility. However, a couple of groundbreaking research articles published thus far, including one published by scientists at the University of Miami Miller School of Medicine, it clearly establishes that the COVID-19 virus enters the human testes during the infection period (Figure 2). This is in contrast with other research studies published regarding the COVID-19 virus and its effects on male fertility. Most research articles, including the one’s focused in this short review article, have focused on sperm parameters and function with little focus on the testes as a whole. Although there is speculation regarding the entry of SARS-CoV-2 via the testes due to ACE2 expression, photographic evidence validates these assumptions, and it is a promising piece of evidence concerning such events. The photographic evidence revealed by Chu et al.9 (Figure 2) clearly depicts the images of SARS-CoV-2 particles isolated in the tissues of the testes and it is of immense significance to this field of research, and it should be explored further.
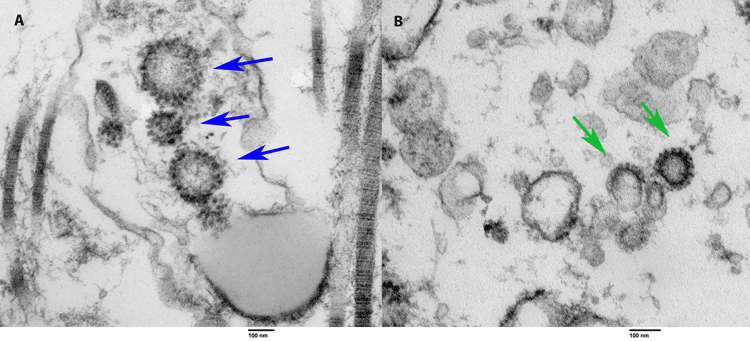
Figure 2 Image retrieved via electron microscopy containing COVID-19 particles. Image on the left containing the blue arrows (A) is tissue taken from the reproductive organs of an alive and once COVID-positive male patient. On the right containing the green arrows (B) is tissue from the reproductive organs collected during an autopsy of a man who died from COVID-19. In both images, the arrows point towards the viral COVID-19 particles which are spiked.10
The COVID-19 virus produces countless threats against the male reproductive system due to the testes’ abundance of angiotensin‐Converting Enzyme‐2 (ACE2), the receptor entry of the virus, which brings about a change in the LH-to-testosterone ratio leading to decreased Leydig cell efficiency, and SARS‐CoV‐2‐associated fever.5 Furthermore, recent studies have signified a gender difference for SARS‐CoV‐2 rates and comorbidity, as well as susceptibility to reproductive harm between the two genders. For instance, proteases such as TMPRSS12 and TMPRSS11B along with the ACE2 gene are present in the male germline/sperm and act as activators for viral infection (including SARS-CoV-2; Aitken.4). These proteases (TMPRSS12 and TMPRSS11B) in the plasma membrane can operate as activating proteases in accordance with the ACE2 gene in order to support viral infection in the testes.4 However, the female oocyte does not express these two proteases, and therefore makes COVID-19 infection in females much less probable and destructive.4
Moreover, in the study conducted by Zavos et al.1 showed a definite potential effect of SARS‐CoV‐2 on male fertility by investigating its effects on the actual ejaculate status. When comparing the spermatogenic parameters of patients non-infected with COVID-19 (Group 1) versus those infected with COVID-19 (Group 2) it was shown clearly a decline in all sperm parameters from the COVID-19 infected group of patients. Baseline values of the patients’ spermatogenic parameters prior to being infected with COVID-19 were used to evaluate the patients’ sperm overall criterion. These same spermatogenic parameters were compared to a second sperm sample collected from the same group of patients 90 days and 110 days (one spermatogenic cycle) post COVID-19 infection, respectively. The data clearly depicts a significant effect on almost all of the sperm parameters assessed and uniquely shows significant effects of the stability of the DNA of the sperm collected from those patients that were infected with SARS-CoV-2. Furthermore, when considering the oxidative stress and DNA damage to the sperm samples from the Group 2 of patients that had COVID-19, one can deduct that such harm directed towards these sperm cells could have significant effects on their abilities to participate in causing adequate fertilization. Subsequently, if fertilization could occur in those patients, one should be aware of the possible abnormalities that could have on the fetus (phenotypic or not) by such damaged sperm fertilizing an oocyte.
Going even further, Zavos et al.1 aimed to explain the correlation between reactive oxygen species and oxidative stress on the sperm cells. As mentioned in the article, an increased generation of free radicals can lead to abnormal spermiogenesis and large amounts of retention in the cytoplasm - a consequence of regenerating reactive oxygen species. This type of oxidative stress can lead to a sperm cell’s loss of motility, decrease in fertilizing ability, and the possible initiation of DNA impairment in the sperm nucleus.2 The way that oxidative damage can affect the sperm’s DNA is not well-known, but, current research demonstrates that in addition to a decreased chance of spontaneous pregnancy,10 and a reduced chance of carrying the fetus to live birth following IVF/ICSI,11,12 early pregnancy loss and morbidity in the offspring, such as childhood cancer, may correlate with such damage. Although the study carried out by Zavos et al.1 did not mention such possibilities, one needs to consider these educated presumptions very seriously and understand that further and more intense studies must be undertaken to evaluate the negative effects of the global COVID-19 pandemic on male fertility.
The data presented in the study by Zavos et al.1 defines a clear association between SARS-CoV-2 and its effects on men’s seminal characteristics. Furthermore, the paper reviews and depicts rather clearly such observations made regarding men’s reproductive health during the current pandemic which should be taken very seriously. Several articles cited in this short review paper aimed to highlight the presence of the SARS-CoV-2 virus in the semen of male patients.5 However, in the study produced by Zavos et al., real raw data is used to support such claims made in various articles regarding the COVID-19 virus’ capacity andability of entering/infecting the testicular parenchymal tissues. This was achieved by observing the generated data that demonstrated decreased sperm quality after one spermatogenic cycle (90 to 110 days post COVID-19 infection) in the patient’s sperm samples. When considering the current research available, it remains evident that SARS-CoV-2 can affect the process of spermatogenesis, the physiology of reproductive tissues, and even the sperm cells themselves (the mature spermatozoon).13,14
If such findings are consistent with future research associated with COVID-19 and male fertility, humans will see themselves becoming less and less likely and/or able to reproduce naturally. With that, more innovative methods such as IVF and sperm donation will have to be used for families that wish to conceive but do not have the means do it on their own. If these processes of conception see themselves becoming more popular, which they will if the findings generated and discussed in this manuscript remain to be true in the future, costs will need to be minimized to ensure everyone’s safety when planning to conceive and establish a family. For instance, there has been an increased number of women lured into sexual assault via third party sperm donation websites. The reason these third party and unprofessional sperm donation entities are even created is because of the expenses that come with carrying out such a process through legitimate, properly licensed medical institutions and companies. With such threats and expenses that come with sperm donation and IVF, and an increasingly infertile population, it is important that everyone can receive the appropriate amount of medical care from a professional without socioeconomic boundaries if they do wish to conceive. These future implications hold serious weight and should not be taken lightly in any medical settings or practices. It is of vital importance that healthcare professionals are aware of these long-term effects that the SARS-CoV-2 pandemic has brought upon us as a species and prepare themselves to help the future generations as much as possible to procreate and perpetuate the human species.
None.
The authors have nothing to disclose.
None.

©2021 Zavos. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.