
Review Article Volume 2 Issue 1
Birth control-current contraception devices and medications: clinical review
Michael Obrowski,1
Regret for the inconvenience: we are taking measures to prevent fraudulent form submissions by extractors and page crawlers. Please type the correct Captcha word to see email ID.

Stephanie Obrowski2
1Assistant Professor of Anatomy, Chief Physician and Surgeon of Wilderness Physicians, Poland
2Medical University of Lodz, President of Wilderness Physicians, Poland
Correspondence: Michael Obrowski, Assistant Professor of Anatomy; 43C Zeligowskiego Street, Apt. 45, Lodz, Poland
Received: February 04, 2015 | Published: March 4, 2016
Citation: Obrowski M, Obrowski S. Birth Control-current contraception devices and medications: clinical review. MOJ Womens Health. 2016;2(1):34-40. DOI: 10.15406/mojwh.2016.02.00022
Download PDF
Abstract
Birth Controlis more properly referred to medically as contraception or fertility control, regardless of what form it takes. Contraception prevents pregnancy by interfering with the normal process of ovulation, fertilization, and implantation. There are different kinds of birth control that act in different ways and at different points in the process. Birth Control has been around since ancient times, first being mentioned in the Egyptian Kahun Papyrus from 1850 B.C. (3,862years ago) and have within them some of the earliest documented descriptions of birth control: the use of honey, acacia leaves and lint to be placed in the vagina prior to intercourse to block sperm. It sounds uncomfortable for both partners, as acacia leaves can be quite sharp and serrated, depending on the species, which is unknown to modern science. We find it necessary to write this report not only because there is a tremendous lack of information amongst women, but even experienced Obstetricians and Gynaecologists often get lost in the myriad of contraceptive devices. Obviously we are aiming this towards our fellow medical colleagues but we will communicate personally with any woman that requires additional knowledge.
Keywords: birth control, contraception, fertility control, fallopian tubes, ovaries, ova, ovulation, uterus, fertilization, implantation, USAID (united states agency for international development), mirena®, implanon®
Purpose of contraception
Every month, a woman’s body begins a process that can potentially lead to pregnancy. As an ovum matures, the mucus that is secreted by the cervix alters its chemistry to be more acceptable to sperm trying to cross the natural barrier to the cervix, known as the mucus plug. The lining of the uterus grows in preparation for receiving a fertilized egg. Any woman who wants to prevent pregnancy must use a reliable form of birth control to interfere with this process.
Contraception is designed to interfere with the normal process and prevent the pregnancy that could result. There are different kinds of birth control that act at different points in the process, from ovulation, through fertilization, to implantation. Each method has its own side effects and risks. Some methods are more reliable than others. There are more different types of birth control available today than ever before. They can be divided into a few groups based on how they work. These groups include:
Hormonal methods
These hormones used to prevent ovulation. Hormonal methods include oral contraceptives (the pill), Depo Provera Injections and finally Implanon®, which is a far advanced form of birth control, designed (in our opinion) to replace Norplant®.
Norplant1-3
- It must be noted that Norplant 1 was removed from the “Contraceptive Regimen” in the USA due to numerous lawsuits in the USA.
- Norplant 1 was completely pulled from the market in 2002 by the manufacturer Wyeth-Aherst, after being forced by the U.S. FDA and after an undisclosed number of out of court settlements.
- Unfortunately this did not cover Europe and Africa, where an efficacious birth control regimen was desperately needed, especially in the African Continent where population growth is increasing at an uncontrolled rate. However, Wyeth-Aherst continued to manufacture Norplant 1 and the USAID (United States Agency for International Development) continued to purchase millions of Norplant I for use on women in the developing world.
- Such women were, after all, easy targets. They lacked the means to fight back legally, and their complaints were brushed off by local health agencies complicit in population control programs.
- Since “Norplant” has become a byword for a dangerous contraceptive drug/device, Norplant II has been given a new name. It will be marketed under the name Jadelle. Of course, a dangerous contraceptive by any other name is still a dangerous contraceptive.
- USAID finally ended its contract with the manufacturer in 2006, after PRI (Population Research Institute, a Non-Profit Organization based in Front Royal, Virginia, USA) called attention to the obvious double standard at work here: “How can the U.S. continue to promote the use of a drug/device overseas, we asked, that are so dangerous they have been taken off the market in the U.S.?”
- Now the same thing is happening all over again. It turns out that the new manufacturer, Bayer HealthCare, has no plans to market Norplant II in the United States.
- USAID has nevertheless signed a contract with the German pharmaceutical company to purchase and distribute the drug/device to its population control partners to implant in poor women around the world.
- Both Norplants (1 and 2) are implanted under the skin of the upper arm. Both contain the same “active ingredient”: levonorgestrel, a synthetic progestin. Like other such steroid-based drugs, it thickens the cervical mucus, sometimes (but not always) inhibits ovulation, and alters the lining of the uterus to prevent implantation. This means that women on Norplant can conceive children, who are then aborted after failing to implant in the uterus.
- The main difference between the two Norplants is a relatively minor one. Norplant I contains six silicon rods containing synthetic progestin, while Norplant II contains only two, albeit larger, rods. In fact the two drug/devices are so similar that when the FDA approved Norplant II way back in 1996, it relied mostly upon Norplant I studies.
Implanon®(etonogestrel implant) 68 mg3-10
- Etonogestrel contraceptive implant, sold under the brandnames Nexplanon and Implanon, is a single-rod subdermal contraceptive implant made by Merck & Co.
- It is inserted just under the skin of a woman’s upper arm and contains etonogestrel.
- The single rod is simply “injected” subdermally.
- Nexplanon/Implanon are a type of long-acting reversible contraception, the most effective form of birth control.
- Nexplanon and Implanon NXT are essentially identical to Implanon except Nexplanon and Implanon NXT have 15 mg of barium sulphate added to the core, so it is detectable by x-ray.
- Nexplanon/Implanon NXT also has a pre-loaded applicator for easier insertion.
- Implanon was first approved for use in Indonesia in 1998, then approved for use in the United States in 2006.
- Subdermal contraceptive implants are now used by 11 million women around the world and approved for use in over 60 countries as of 2003.
Device description
- Nexplanon/Implanon consists of a single rod made of ethylene vinylacetate copolymer that is 4 cm long and 2mm in diameter.11 It is similar to a matchstick in size.
- The rod contains 68 mg of etonogestrel (sometimes called 3-keto-destrogestrel), a type of progestin.
- It is implanted in a quick, simple procedure by a trained physician in the non-dominant arm, approximately 3 to 4 cm. above the medial olecron process, avoiding the sulcus, where numerous nerves, major arteries and veins lie.
- The clinician MUST make sure Implanon is placed subdermally and should not be performed without prior training of the physician.
- Peak serum etonogestrel concentrations have been found to reach 781-894 pg/mL in the first few weeks, gradually decreasing to 192-261 pg/mL after 1 year, 154-194 pg/mL after 2 years, and 156-177 pg/mL after 3 years, maintaining ovulation suppression and contraceptive efficacy.
- Serum levels maintain relatively stable through 36 months, which implies that the method may be effective for longer than 3 years.
Barrier methods
These methods work by physically preventing the sperm from getting to and fertilizing the egg.
- Barrier methods include the condom, diaphragm, cervical cap and the contraceptive vaginal sponge.
- The condom is the only form of birth control that also protects against sexually transmitted diseases, including HIV.
- However patients must be informed that the ONLY form of birth control that is 100% effective against pregnancy and sexually transmitted diseases is ABSTINENCE.2,12-15
- Doctors just do not want to talk to patients about this but it is a sad part of our 21st Century existence. HIV became rampant in the late 20th Century (1980’s) and killed and infected thousands of people.
- We ALWAYS advise our patients that if they are even remotely thinking of having any personal contact with a partner, is that they go together and have a full STD Panel!
- It may upset their potential sexual partner, but better to be safe than sorry, or dead.
- Remember, we are not only talking about HIV/AIDS but also Hepatitis and Herpes Virus - which are incurable, horribly contagious and often the disease process can deteriorate to the point of needing a liver transplant, a life-altering procedure in many ways.
- Currently, we know of one medical student (who you would think would know better), we’ll call him John; who has a raging case of Herpes Simplex Virus. This student, because of alcohol, bad judgement, using prostitutes, stupidity or a combination of all four, is now doomed forever to be a Herpes Carrier and a vector for spreading this terrible disease which can cause many medical problems, including sterility.
Contraceptive sponge
Combines barrier and spermicidal methods to prevent conception (Figure 1).
- Three brands are currently marketed: Pharmatex, Protectaid and Today. Pharmatex is marketed in France and the province of Quebec; Protectaid in the rest of Canada and Europe; and Today in the United States.16
- Sponges work in two ways. First, the sponge is inserted into the vagina, so it can cover the cervix and prevent any sperm from entering the uterus.
- Secondly, the sponge is produced saturated with spermicide already inside of it, which is used to prevent the sperm from moving.17
- The sponges are inserted vaginally prior to intercourse and must be placed over the cervix to be effective. Sponges provide no protection from sexually transmitted diseases (STD’s).18

Figure 1 Action and Placement of Contraceptive Sponge
Effectiveness
- The manufacturer of the Today sponge reports effectiveness for prevention of pregnancy of 89% to 91% when used correctly and consistently. When packaging directions are not followed for every act of intercourse, effectiveness rates of 84% to 89% are reported.19,1 Other sources cite poorer effectiveness rates for women who have given birth: 74% during correct and consistent use, and 68% during typical use.1
- Studies of Protectaid have found effectiveness rates of 77% to 91%.2,12
- Studies of Pharmatex have found perfect use effectiveness rates of over 99% per year.20 Typical use of Pharmatex results in effectiveness of 81% per year.21
- Sponges may be used in conjunction with another method of birth control such as condoms to increase effectiveness.
Use
- To use the Today sponge, it must be run under water until thoroughly wet, about 2 tablespoons. The water is used as a mechanism to activate the spermicide inside the sponge.No extra spermicide is needed. The Protectaid and Pharmatex sponges come ready to use.
- The sponge can be inserted up to 24 hours before intercourse. It must be left in place for at least six hours after intercourse. It should not be worn for more than 30 hours in a row.
- The sponge should never be reused once it has been removed after having sexual intercourse.
History
- The devices have had periods of unavailability in some markets since being introduced. All three brands are currently available outside their normal marketing areas through internet retailers.
Today sponge
- The Today Sponge was developed beginning in 1976 and introduced in the United States in 1983. Today was removed from the market in 1994. Following several delays, the Today brand became available again in Canada in March 2003, and in the U.S. in September 2005.
- After the manufacturer’s parent company declared bankruptcy in 2007, the brand was off the market until being relaunched in 2009.
Pharmatex sponge
- The Pharmatex sponge was introduced in France and the Quebec province in Canada in 1984.
- Protectaid Sponge
- The Protectaid sponge was introduced in Canada in 1996 and in Europe in 2000.
Spermicide
- Sponges are a physical barrier, trapping sperm and preventing their passage through the cervix into the female reproductive system. The spermicide is an important component of pregnancy prevention; each brand offers a different formula.
- The Today sponge contains 1,000 milligrams (mg) of nonoxynol-9.Protectaid contains 5,000 mg of the F-5 gel, with three active ingredients (6.25 mg of nonoxynol-9, 6.25 mg of benzalkonium chloride, and 25 mg of sodium cholate).Pharmatex contains 60 mg of benzalkonium chloride.
- Side effects
Some people are allergic to the spermicide used in the sponge. Women who use contraceptive sponges have an increased risk of yeast infection and urinary tract infection. Improper use, such as leaving the sponge in too long, can result in toxic shock syndrome.
The Today sponge contains the spermicide nonoxynol-9, which may contain certain risks for those using the sponge multiple times a day, or for those at risk for HIV. In these cases, nonoxynol-9 can irritate the tissue, which leads to an increased risk of HIV and other sexually transmitted infections.
Spermicides
These medications kill sperm on contact. Most spermicides contain nonoxynyl-9. Spermicides come in many different forms such as jelly, foam, tablets, and even a transparent film. All are placed in the vagina. Spermicides work best when they are used at the same time as a barrier method.
Intrauterine devices
Intrauterine contraceptive devices (IUDs) are inserted into the uterus by a physician properly trained in the technique, where they stay from one to 10 years (Table 1). An IUD prevents the fertilized egg from implanting in the lining of the uterus, and may have other effects as well. The newest, most efficacious in our opinion, is the Spiral T, the most often cited one is Mirena® .which will be discussed in a separate section on implantable IUD’s (Intrauterine Devices).
Copper IUD |
LNG-IUS (Mirena) |
Cost less ($50-$180) |
Cost more (?insurance) |
No hormones/natural cycles |
5% removed for hormone S/E |
20-5o% more bleeding and pain |
Irreg bleeding 1st 3m, 50% no periods in iy |
I pregnancy/100wy |
I pregnancy/1000wy |
Tubal ligation (Sterilization)
Tubal sterilization is a permanent form of contraception for women. Each fallopian tube is either tied or burned closed. The sperm cannot reach the egg, and the egg cannot travel to the uterus.
Usually the sequelae of the surgery are irreversible and permanent.
Vasectomy
Is the male form of sterilization, and should also be considered permanent. In vasectomy, the vas defrens, the tiny tubes that carry the sperm into the semen, are cut and tied off. Thus, no sperm can get into the semen.
A newer and somewhat controversial form of birth control is emergency contraception. This type is used after unprotected intercourse and sometimes is referred to as the “morning-after pill”.
Unfortunately, there is no perfect form of birth control. Only abstinence (not having sexual intercourse) can protect against unwanted pregnancy with 100% reliability.
The failure rates, which mean the rates of pregnancy, for most forms of birth control are quite low.
However, some forms of birth control are more difficult or inconvenient to use than others. In actual practice, the birth control methods that are more difficult or inconvenient have much higher failure rates because they are not used regularly or as prescribed.
Description
Most forms of birth control have one thing in common. They are only effective if used faithfully. Birth control pills will work only if taken every day; the diaphragm is effective only if used during every episode of sexual intercourse. The same is true for condoms and the cervical cap. Some methods automatically work every day. These methods include Depo Provera, Implanon, the IUD and tubal sterilization.
There are many different ways to use birth control. They can be divided into several groups:
- Oral - Birth control pills must be taken by mouth every day (Figure 2).
- Injected - Depo Provera is a hormonal medication that is given by injection every three months.
- Implanted - Norplant and the newer, far advanced Implanon are a long-acting hormonal form of birth control that is implanted under the skin of the upper arm.
- Vaginal - Spermicides, sponges and barrier methods work in the vagina.
- Intra-uterine Device - The IUD is inserted into the uterus (Figure 3).
- Surgical - Tubal sterilization or a vasectomy (in males) is a form of surgery. A doctor must perform the procedure in a hospital or surgical clinic. Many women need general anesthesia.
The methods of birth control differ from each other in the timing of when they are used. Some methods of birth control must be used specifically at the time of sexual intercourse (condoms, diaphragm, cervical cap, spermicides). Emergency contraception must be started as soon as possible after intercourse and no more than 72 hours after. All other methods of birth control (hormonal methods, IUDs, tubal sterilization) must be working all the time to provide protection.
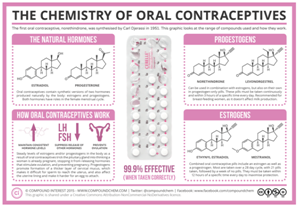
Figure 2 Chemistry of Oral Contraceptives.
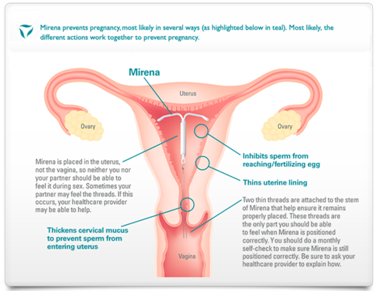
Figure 3 Action and Placement of Mirena®
Precautions
There are risks associated with certain forms of birth control. Some of the risks of each method are listed below:
- Birth control pills -The hormone (estrogen) in birth control pills can increase the risk of heart attack in women over 35, particularly those who smoke. Certain women cannot use birth control pills.
- IUD -The IUD can increase the risk of serious pelvic infection. The IUD can also injure the uterus by poking into or through the uterine wall. Surgery might be needed to fix this.
- Tubal Ligation (Sterilization) -”Tying the tubes” is a surgical procedure and has all the risks of any other surgery, including those associated with anesthesia, as well as infection and bleeding.
- Emergency Contraceptive Pills- should not be used regularly for birth control (Table 2). They can interrupt the menstrual cycle and are not 100% effective.
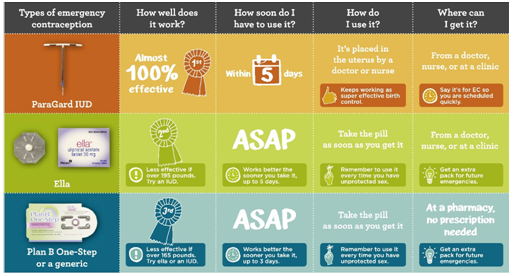
Table 2 Emergency Contraception. Birth control that works after sex
Preparation
No specific preparation is needed before using contraception. However, a woman (and the prescribing physician) must confirm that she is not already pregnant before using a hormonal method or having an IUD placed.
Aftercare
No aftercare is needed.
Risks
Many methods of birth control have side effects. Knowing the side effects can help a woman to determine which method of birth control is right for her.
- Hormonal methods: The hormones in birth control pills, Depo Provera, and Norplant can cause changes in menstrual periods, changes in mood, weight gain, acne, and headaches. In addition, it may take many months to begin ovulating again once a woman stops using Depo Provera or Norplant.
- Barrier methods: A woman must insert the diaphragm in just the right way to be sure that it works properly. Some women get more urinary tract infections if they use a diaphragm. This is because the diaphragm can press against the urethra, the tube that connects the bladder to the outside.
- Spermicides: Some women and men are allergic to spermicides or find them irritating to the skin.
- IUD: The IUD is a foreign body that stays inside the uterus, and the uterus tries to get it out. A woman may have heavier menstrual periods and more menstrual cramping with an IUD in place.
- Tubal ligation (Sterilization): Some women report increased menstrual discomfort after tubal ligation. It is not known if this is related to the tubal ligation itself.
There is no perfect form of birth control (Figure 4). Every method (except Abstinence) has a small failure rate and side effects. Some methods carry additional risks. However, every method of birth control can be effective if used properl (Table 3).22-34
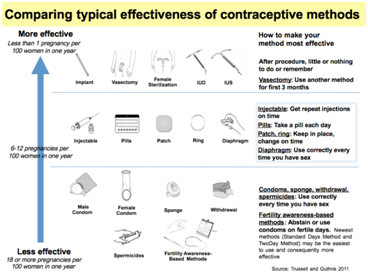
Figure 4 Comparing Typical Effectiveness of Contraceptive Methods.
Skyla |
Mirena |
ParaGard |
Works by releasing a low amount of hormone (1 4 micrograms per day) |
Works by releasing a low amount of hormone (20 micrograms per day) |
Works because it has a small amount of copper in it-no hormones |
Plastic frame of the IUD is 1.1 by 1.2 inches |
Plastic frame of the IUD is 1.3 inches square |
Plastic frame of the IUD is 1.3 by 1.4 inches |
Tube used to place the IUD is 0.15 inches wide |
Tube used to place the IUD is 0.19 inches wide |
Tube used to place the IUD is 0.16 inches wide |
Can be used for up to 3 years |
Can be used for up to S years |
Can be used for up to 12 years |
Table 3 IUD Brand comparisons
Human studies
No human patients were used in the development and write-up of this paper.
Acknowledgements
Conflict of interest
The author declares no conflict of interest.
References
- Hanson SJ, Burke, Anne E. Fertility control: contraception, sterilization, and abortion. In: Hurt K et al, editors. The Johns Hopkins Manual of Gynecology and Obstetrics. 4th ed. Wolters Kluwer Health, Philadelphia, USA: Lippincott Williams & Wilkins; 2010. p. 382–395.
- WHO. Family planning: A global handbook for providers: Evidence-based guidance developed through worldwide collaboration. Geneva, Switzerland: World health organization department of reproductive health and research; 2011.
- WHO. Family planning: A global handbook for providers. Evidence-based guidance developed through worldwide collaboration. Geneva, Switzerland: WHO and center for communication programs; 2011. p. 260–300.
- Implanon® (etonogestrel implant) 68mg.
- Physician Prescribing Information for Implanon
- Funk S, Miller MM, Mishell DR, et al. Safety and efficacy of Implanon, a single-rod implantable contraceptive containing etonogestrel. Contraception. 2005;71(5):319–326.
- Flores JB, Balderas ML, Bonilla MC, et al. Clinical Experience and acceptability of the etonogestrel subdermal contraceptive implant. Int J Gynaecol Obstet. 2005;90(3):228–233.
- Raj K, Gupta S, Cotter S. Experience with Implanon in a northeast London family planning clinic. Eur J Contracept Reprod Health Care. 2004;9(1):39–46.
- Booranabunyat S, Taneepanichskul S. Implanon use in Thai women above the age of 35years. Contraception. 2004;69(6):489–491.
- Zheng SR, Zheng HM, Qian SZ, et al. A long-term study of the efficacy and acceptability of a single-rod hormonal contraceptive implant (Implanon) in health women in China. Eur J Contracept Reprod Health Care. 1999;4(2):85–93.
- Killick SR, Leary C, Trussell J, et al. Sperm content of pre-ejaculatory fluid. Hum Fertil (Camb). 2011;14(1):48–52.
- Chin HB, Sipe TA, Elder R, et al. The effectiveness of group-based comprehensive risk-reduction and abstinence education interventions to prevent or reduce the risk of adolescent pregnancy, human immunodeficiency virus, and sexually transmitted infections. Am J Prev Med. 2012;42(3):272–294.
- Abstinence Planned Parenthood 2009 Retrieved 2009-09-09.
- Fortenberry JD. The limits of abstinence-only in preventing sexually transmitted infections. J Adolesc Health. 2005;36(4):269–270.
- Ott MA, Santelli JS. Abstinence and abstinence-only education. Curr Opin Obstet Gynecol. 2007;19(5):446–452.
- Ectopic Pregnancy Is a Possibility When Emergency Contraception Fails. Health & Medicine Week March; 2004. 222 p.
- Handbook of Early Pregnancy Care. London, UK: CRC Press; 2006. p. 149–154.
- Rowan SP, Someshwar J, Murray P. Contraception for primary care providers. Adolesc Med State Art Rev. 2012;23(1):95–110.
- Arthur T. Manual of Obstetrics. 7th ed. Philadelphia, USA: Lippincott; 2007. p. 265–268.
- Duffy K, Lynch DA, Santelli J. Government support for abstinence-only-until-marriage education. Clin Pharmacol Ther. 2008;84(6):746–748.
- Black AY, Fleming NA, Rome ES. Pregnancy in Adolescents. Adolesc Med State Art Rev. 2012;23(1):123–138.
- NWHRC. Contraception: Overview. National women's health resource center. Health center-contraception; 2004.
- WHO. Medical eligibility criteria for contraceptive use. 4th ed. Reproductive Health and Research. Geneva, Switzerland: World Health Organization; 2009. p. 91–100.
- Jones RK, Fennell J, Higgins JA, et al. Better than nothing or savvy risk-reduction practice? The importance of withdrawal. Contraception. 2009;79(6):407–410.
- Cheng L, Che Y, Gülmezoglu AM. Interventions for emergency contraception. Cochrane Database Syst Rev. 2012;8:CD001324.
- Richardson AR, Maltz FN. Ulipristal acetate: review of the efficacy and safety of a newly approved agent for emergency contraception. Clin Ther. 2012;34(1):24–36.
- ARHP. Update on Emergency Contraception. Association of reproductive health professionals; 2011.
- Cleland K, Zhu H, Goldstuck N, et al. The efficacy of intrauterine devices for emergency contraception: a systematic review of 35 years of experience. Hum Reprod. 2012;27(7):1994–2000.
- Glasier A, Cameron ST, Blithe D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception. 2011;84(4):363–367.
- Kripke C. Advance provision for emergency oral contraception. American Family Physician. 2007;76(5):654.
- Shrader SP, Hall LN, Ragucci KR, et al. Updates in hormonal emergency contraception. Pharmacotherapy. 2011;31(9):887–895.
- Dual protection against unwanted pregnancy and HIV/STDs. Sex Health Exch. 1998;(3):8.
- Michael O, Stephanie O. Hyperemesis gravidarum - a serious issue during pregnancy: In-depth clinicalreview and treatment modalities. MOJ Women’s Health. 2015;1(2):00009.
- Michael O, Stephanie O. Stopping massive, uncontrollable uterine bleeding post-caesarean section: a case report. MOJ Women’s Health. 2015;1(2):00006.

©2016 Obrowski, et al. This is an open access article distributed under the terms of the,
which
permits unrestricted use, distribution, and build upon your work non-commercially.


