MOJ
eISSN: 2641-9300


Short Communication Volume 1 Issue 5
Department of Tumor Biotherapy, N.N. Blokhin Russian Cancer Research Center, Moscow, Russian
Correspondence: Samoylenko IV, Department of Tumor Biotherapy, NN Blokhin Russian Cancer Research Centre, 115478, 23 Kashirskoe shosse, Moscow, Russia
Received: August 20, 2018 | Published: September 5, 2018
Citation: Samoylenko IV, Demidov LV. Prolonged survival of the patient with metastatic melanoma sequentially treated with different therapeutics. MOJ Tumor Res. 2018;1(5):153-156. DOI: 10.15406/mojtr.2018.01.00034
Despite the dramatic changes in treatment options during last five years metastatic melanoma remains a disease with poor prognosis leads to 2-years survival rate not more than fifty per cent. New drugs such as BRAF inhibitors and immune checkpoint inhibitors are able to shift median overall survival rate from 12 months to more than 24 months.1,2 Randomized controlled studies have shown that all drugs either BRAF inhibitors or immune checkpoint inhibitors works much more better in patients with good performance status, low LDH level and low tumour burden.3,4 In some cases practical oncologists conclude that metastatic melanoma patients with poor prognostic features have no real benefit from treatment, could developed unacceptable toxicity and should be underwent best supportive care only. We would like to present an excellent case of prolonged survival of patient with high tumour load and initially very poor performance status who was sequentially treated with different anticancer regimens.
Patient, female white lady, 49 years old, was referred to our clinics in March of 2012 with complaints to fatigue, intermittent febrile (38.2-38.5 Celsius) axillary temperature, cough, and findings on ultrasound of her liver. Her disease history includes excision of the skin melanoma on the right side of her neck and simultaneous right-sided elective neck dissection in October 2010. According to pathology report skin tumour was represent with ulcerated pigmented melanoma 5mm Breslow thickness, Clark level IV. Among sixteen investigated neck lympnodes no metastasis were found. Patient advised for follow up, no adjuvant treatment was prescribed. Patient missed almost all follow up recommendations and came to her local oncologist in March 2010 complaining to general malaise, subfebrile temperature and weight loss (9kg for 3 months). Routine examination with abdomen ultrasound and chest x-ray revealed large metastatic lesions in the liver and lungs.
Important point of the medical history of our patient is long history of schizophrenia, which manifested for the first time when patient was twenty-two years old, well controlled along her life, but exacerbated from time to time. Cognitive function and criticism are on very good level. Patient works as a designer, has got her own family (son and husband). There was no any evidence of cancer or melanoma among her close relatives. At the time of her first visit to our clinic in March of 2012 she had Eastern cooperative group (ECOG) performance status 2, height 161cm, weight 51kg, BMI 19.7kg/m2. Disease staging showing multiple lung metastasis (with maximum diameter 40mm), mediastinal lymphnodes metastases (maximum diameter 35mm), single liver metastasis (100mmx90mm) (Figure 1), LDH level was 3224 U/l (≈7 upper limits of normal, ULN), S100 protein 6.95 mcg/L. Brain contrast enhanced MRI revealed no evidence of disease, as well as a bone scan with 99mTc. For diagnosis confirmation liver metastasis was biopsied, activating BRAF V600E mutation was found. At that moment we did not have any BRAF inhibitor registered in Russia as well as active clinical trials, and chemotherapy or best supportive care were only the options available for this patient.
Chemotherapy with DTIC, CCNU, CDDP and TNF-alfa was prescribed. Since April to May 2012 she received one cycle of recombinant tumor necrosis factor–thymosin alfa conjugate (Refnot) 100’000 IU per day subcutaneously (s.c.) days 1-5, week 1-4, DTIC 400 mg intravenously (i.v.) days 1-5, CCNU 80 mg per os, CDDP 20 mg/m2 days 1-5 i.v. After the cycle patient became ECOG =3 and felt worse. Unscheduled tumour assessment revealed obvious disease progression: tumour metastasis enlarged to 140 mm x 110 mm, most of lung metastases also enlarged with a highest diameter up to 50 mm. LDH level increased up to 5880 U/l (≈13 ULN), S100 protein 7.39 mcg/L (Figure 1). Patient was included in expanded access to vemurafenib in another country in June 2012. Dramatic clinical improvement and partial response was achieved after first two months of vemurafenib administration. Modest adverse events (follicular keratosis of the skin, keratopapillomas, joint pain) were self-limiting and disappeared during six months of treatment. It should be noted, that since October 2012 to November 2012 treatment was temporary interrupted due to lack of the access to vemurafenib. Tumor assessment performed just before and month ago after treatment interruption revealed enlargement of some metastatic lesions more than in 20 per cents in longest diameters. Nevertheless treatment with vemurafenib was re-challenged and disease control was achieved.
Disease progression (new metastatic lesion) was documented in May 2013. Nevertheless patient ECOG status came up to 0, no complaints were at the moment of tumor assessment. We suggest a palliative chemotherapy for this patient and surprisingly patient developed a partial response (metastatic lesions shrink more than 30 per cent). Since June 2013 to October 2013 patient received five cycles of paclitaxel 225mg/m2 day 1 and carboplatin AUC=6, cycle 21 days, treatment was discontinued due to toxicity (sever neutropenia, anemia, and thrombocytopenia) and partial response was confirmed with two sequential CT scans. Patient ECOG status was 1 and Grade 2 peripheral neuropathy was only the compliant. Nevertheless the overall tumor volume continued to decrease. At that moment we have had expanded access program to ipilimumab and since November 2013 up to January 2014 patient was underwent 4 infusions of ipilimumab 3mg per kg. There were no any adverse events during and after ipilimumab treatment registered, tolerability was excellent and we continued to follow up patients after this induction period. Sequential CT scans showed stable disease course until October of 2014, when one retroperitoneal lymphnode became to grow rapidly.
Patient was re-treated with paclitaxel and carboplatin (three cycles), no response observed, afterwards patients was undergone re-induction of ipilimumab (January 2015–March 2015), no response was observed, new lung lesion was detected, retroperitoneal lymphnodes also enlarged (Figure 1). Patient kept her good performance status, had no any new complaints, chemotherapy related adverse reactions regressed. Patient was included in nivolumab trial and since August 2015 she has been receiving nivolumab 3mg/kg every two weeks. No treatment related adverse events were detected. According to regular tumor assessments there no any changes in metastatic lesions size, no new lesions observed.
We have observed a patient with extremely poor prognostic features at time when her metastatic melanoma was discovered. New drugs sequentially administered to our patient dramatically change her natural disease course and lead to prolonged survival. In this case we have observed some interesting features that are very debatable in literature. First of all we of course have to highlight that chemotherapy could not be an option for the first line treatment of BRAF-positive patient. Initiating of BRAF-inhibitor dramatically improved patient’s performance status as well as decreased the tumor load. We also observed that interruption of treatment with BRAF-inhibitor in our patient with incomplete response lead to obvious disease progression. Re-introduction of BRAF inhibitor could be effective and stable disease could be achieved. The next problem that we faced with was an acquired resistance to BRAF inhibitor. Treatment options for patients progressed on BRAF/MEK inhibitors are limited and up to the moment usually include anti-PD1 therapies or combinations anti-PD1/anti-CTLA4, or best supportive care. Almost nobody of our colleagues form Europe or United States would not like to offer palliative chemotherapy in this setting due to poor effectiveness, toxicity or considerations about “archaic regimens”. Nevertheless we have to stress that in large part of the world anti-PD1 therapy is unavailable for most patients because of their high price. It is also very well known that anti-PD1 drugs is less effective in second line and seems to be used for first line treatment regardless of patient BRAF-status. So we will often face with patient acquired resistance to BRAF/MEK inhibitor who already received all kinds of immunotherapy. And we have observed a very nice partial response on chemotherapy in initially chemo-resistant patient probably facilitated with previous BRAF-inhibitor treatment. This was a patient who shift us for retrospective analysis recently presented on ASCO.5 Very debatable decision was ipilimumab induction and re-induction in heavy pretreated patient (including BRAF inhibitor) with large tumor masses in the liver and in the lungs. Recently published paper about prognostic scores predicting ipilimumab treatment outcomes strongly advises do not initiate ipilimumab treatment in patient with high tumor load, high LDH level, poor performance status etc.4 If we would apply these criteria to our patient we would never start with ipilimumab and probably miss a durable (approximately 10months) stable disease course (Figure 2) (Figure 3).
One more question rising now is for how long we should continue treatment with anti-PD1 agent nivolumab. We have not seen any changes in performance status or tumor size changes on CT scans. According to clinical trial protocol this timeframe is limited with two years of treatment but in real life this decision will be very hard when taking into account patients adherence to treatment and treatment costs. Recently presented on ASCO 2016 KEYNOTE-001 data demonstrates safety of treatment discontinuation in patients with complete response on anti-PD1 agent pembrolizumab6 whereas we still need more data for patients who had long-lasting stabilization on incomplete response.
In conclusion we want to highlight that dramatic changes in melanoma landscape treatment during last five years are able now to prolong survival for patient with metastatic melanoma and very poor prognostic features. Most effective treatment should be delivered to patient as soon as possible. It should be noted, that some patients who progressed on BRAF-inhibitors (as first or second line treatment) could benefit from chemotherapy with paclitaxel and carboplatin. Patient with high tumor load and poor prognostic features should not be rejected from immunotherapy in favor to best supportive care as those patients also could achieve clinical benefit form anti-CTLA4 and anti-PD1 therapy. More data from clinical trials are warranted to select the best treatment options for patient with BRAF-positive metastatic melanoma and high tumor load (Figure 4).
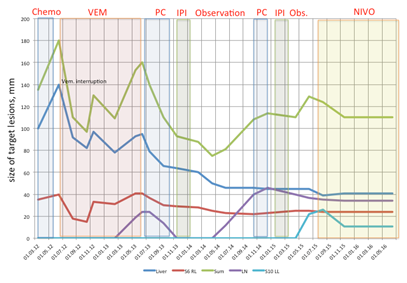
Figure 2 Summary of melanoma metastases size dynamics over the course of patient treatment.
Abbreviations: chemo, chemotherapy with tumor necrosis factor–thymosin alfa conjugate (Refnot) 100’000 IU per day subcutaneously (s.c.) days 1-5, week 1-4, DTIC 400 mg intravenously (i.v.) days 1-5, CCNU 80 mg per os, CDDP 20 mg/m2 days 1-5 i.v.; VEM, vemurafenib; PC, chemotherapy with paclitaxel 225 mg/m2 day 1 i.v. + carboplatin AUC 6; IPI, ipilimumab 3 mg/kg i.v. every 21 day, 4 infusions; Obs., observation; NIVO, nivolumab 3 mg/kg every 14 day, still on treatment; Liver, liver metastasis; S6 RL, metastasis in the segment six of the right lung; Sum, sum of the longest diameters of target lesions; LN, lymph node in the hepatic hilum; S10 LL, metastasis in the segment ten of the left lung.
|
May 2013 |
|
September 2013 (left image) and November 2013 (right image) |
|
June 2015 |
|
Aug 2017 (full metabolic response) |
|
AUGUST 2018 |
Figure 4 Patients scan in different time points.
None
The author declares there is no conflict of interest.

©2018 Samoylenko, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.