MOJ
eISSN: 2641-9300


Case Report Volume 1 Issue 2
Department of Skin and Veneral Diseases IM Sechenov First Moscow State Medical University (Sechenov University), Russian Federation, Russia
Correspondence: Snarskaya ES, Department of Skin and Veneral Diseases of IM Sechenov First Moscow State Medical University (Sechenov University), Moscow, 119991, Russian Federation, Russia
Received: February 14, 2018 | Published: March 12, 2018
Citation: Snarskaya ES, Olisova OY, Ibrahim A, et al. Modern approaches to the treatment of basal cell carcinoma from dermatologists’ point of view. MOJ Tumor Res. 2018;1(2):48 – 51. DOI: 10.15406/mojtr.2018.01.00010
The annual analysis of the disease incidence has indicated that the most frequent malignant epithelial skin tumor is basal cell carcinoma (BCC). BCC is characterized by a variety of clinical forms, locally destructive growth and a high relapse potential with the risks of transformation into metatypical or squamous cell skin cancer. Thus, the article deals with the modern approaches to the treatment of BCC, particularly aggressive (cryotherapy, curettage, electric dissection, Mohs surgery and microsurgery, radiosurgery), therapeutic (laser and radio-wave therapy, PDT, retinoids, chemotherapy, imiquimod, 5 fluorouracil, intralesional cytokines) and innovative methods. The research provides detailed information on the advantages and disadvantages of these approaches and proves that the use of combined methods of BCC therapy appeared to be the most effective treatment.
One of the main criteria in assessing the diagnosis and providing medical care to patients with the skin cancer in public health facilities of the Russian Federation is an indicator of the progressive disease (metastatic tumors, nodular-ulcerative and metatypical variants of basal cell carcinoma of high stages T3-4), which for the last seven years remains above 30%.1‒4 Today, there are neither common approaches and adequate methods of pathogenic therapy of advanced, inoperable forms of the disease nor primary care professionals able to control therapeutic routes of these patients.2,5 An important step towards improving early diagnosis of malignant skin tumors is compliance with the principle of oncological alertness, which requires not only a high level of awareness of the symptoms of precancer, but also the ability to correctly and timely organize an examination and treatment of the patient in early stages of malignant neoplasm.1‒5 This is especially important given the high frequency of registration of advanced skin tumors due to the lack of alertness among doctors of various specialties at the primary prophylactic and polyclinic divisions. According to State Statistics Committee of the Russian Federation No. 49, in the analysis of the malignant skin tumors incidence in the various parts of Russia in the past seven years the incidence rate increased 1.1 times (from 309.9 to 333.0 per 100,000 people) and the average Russian incidence rate exceeded in a number of areas and is even 418.5 per 100,000 people. The results indicate the need to improve preventive examinations in order to timely identify and adequately treat skin neoplasm, and to create adequate conditions for specialized care and prophylactic examination of patients with skin cancer.4 According to the annual analysis of the disease incidence, the most frequent (more than 70%) malignant epithelial skin tumor is basal cell carcinoma (synonym: basaloma). BCC is a slowly growing malignant epithelial tumor characterized by a variety of clinical forms, histological types, with predominant localization in cosmetically important areas – facial skin (80%) and areas chronically exposed to ultraviolet radiation3,5 with rare metastasis, BCC is specified by locally destructive growth and ability to significantly destroy tissues, including cartilage and bone structures in the localization zone. Basal cell carcinoma also has a high relapse potential, as well as an increase in the degree of aggressiveness in the long-term existence, with high risks of transformation into metatypical (MBCC) or squamous cell skin cancer (SCC).3
The relapse incidence of basal cell carcinoma still remains high, which makes it necessary to identify and develop new methods of treatment that will ensure no relapse and a better cosmetic effect. To evaluate the prognosis of different treatment methods effectiveness and the biological behavior of BCC, the clinical form and the corresponding histomorphologic tumor type are of great importance. Most of the primary sites of BCC are represented by superficial and micronodular (synonym: nodular) forms, rarely by morpheaform and extremely rarely by Pinkus tumor. Superficial and micronodular forms of BCC corresponding to T1N0M0 (up to 2 cm in diameter) with a simple histological type of structure (multicentric, solidly compact) are regarded as tumors with a low risk of relapse and progression.2,5,6
Considerable experience has been accumulated in the use of aggressive methods of treatment of skin neoplasm: cryotherapy, curettage, electric dissection, Mohs surgery and microsurgery, radiosurgery. These methods are widespread, but they have a number of contraindications and a quite high percentage of relapse, depending on the method. The modern therapeutic strategy of BCC is innovative pathogenetically substantiated local or systemic drug effects on the process of carcinogenesis. These methods are often combined with each other, thus providing a good therapeutic and cosmetic effect, reducing treatment and rehabilitation period. These include:
a. laser and radio-wave therapy;
b. photodynamic therapy (PDT);
c. retinoids (systemic and topical);
d. chemotherapy (systemic and topical);
e. imiquimod, 5 fluorouracil (topical);
f. intralesional cytokines (IFN-α2, interleukin).
Nowadays one of the innovative methods of BCC treatment is the use of immunomodulatory therapy, in particular, cytokines. Based on the clinical immunomorfological monitoring of 62 patients with BCC, we developed and patented the method of BCC treatment with recombinants Interferon-α2 (reaferon, intron A). Nodular, nodular-ulcerative, morpheaform and any relapsing forms of BCC, T2-4N0M0 (more than 2 cm in diameter) with location in the H-zone of the face and characterized by aggressive types of histological structure (morphea, fibroepithelial, infiltrating-diffuse, basal squamous) represent a big problem for the choice of an adequate therapy, due to the high risks of relapse.7,8 However none of the methods are perfect for the treatment of all types of tumors.2,6,9,10 The highest risk of relapse is during the first 2 years after treatment. So after treatment the relapse of BCC is 1% with primary site and 5.6% with recurrent.10,11 After surgical treatment, according to the literature, in general, the relapse rate of BCC varies from 2 to 41.4%.2,9 The most effective in the world is the micrographic surgery developed by F.E. Mohs, which guarantees 96-98% of recovery from primary BCC and 90-94% of recovery from recurrent BCC;2,8,10 however, it is rarely used in Russia, because it is quite expensive and time-consuming. Micrographic surgery according to Mohs is the gentlest method of surgical intervention that provides controlled serial microscopic examination of tissues taken directly from the patient. The tissue is gradually removed according to a special scheme until the signs of the tumor cease to be determined, the tissue can be used for freezing, obtaining slices and subsequent microscopy. The operation can consist of several consecutive cycles.12 Most often this method is used in the treatment of facial tumors and other cosmetically important localizations, as well as in morpheaform, morphea-like, infiltrating, recurrent BCC, resistant to other types of treatment.12,13 Cryodestruction is the most common and inexpensive outpatient method of therapy with 1-1.5 cm margin of normal skin.9,13 It is indicated for small tumors up to 2 cm in diameter in various localizations, including recurrent after radiation treatment and immunotherapy, with development of neoplasm in scars, primary multiple lesions.13,14,15
This method can be used in elderly patients with the presence of somatic pathology. Cryosurgery is not recommended in macronodular, infiltrating, morphea-like and morpheaform BCC,9,13‒15 as well as in localization of lesions in the areas with the underlying cartilaginous tissue.2,14,15 Multiple freezing of tissues ensures the death of tumor cells by necrosis, as well as the development of humoral and cell-mediated body response to cryotherapy, which slows down the further growth of the tumor.13 The disadvantages of cryodestruction are significant inflammatory reactions and a long period of healing. According to our data, recurrence during 5 years of follow-up after cryotherapy of primary BCC is 4-7.5% and of recurrent is from 13 to 22%.2,5,9 Electro-coagulation (diathermocoagulation) and curettage are currently used very rarely, usually in small (up to 1 cm in diameter) exophytic tumor and its insignificant infiltration. Burning is performed by special electrodes using high-frequency alternating current, with 5-6 mm margin of normal skin.14,16 Curettage is the process of abrasion after anesthetizing the tumor with Volkmann spoon. These techniques are most often used in combination with cryotherapy and radiotherapy.13 According to literature, the relapse use with the use of curettage and electro-dissection is 9.5% in a risk-free localization and 16.3% in a high-risk localization (ear area, nasolabial triangle, periorbital area). If the size of BCC site is up to 5 mm in diameter, the relapse rate is 8.5%, and at 20 mm and more it increases to 19.8%.17,18 Radiation therapy is recognized as the second line of therapy in BCC and used in cases of solitary tumors up to 4-5 cm in diameter.2,5,18,19 Tumor cells death results from the damage of chromosome apparatus and inhibition of the mitotic activity of proliferating cells. Non-proliferating cells have a low radiosensitivity and easily repair damage, giving rise to new cells. This method has a number of significant limitations (multiple sites, the presence of severe somatic pathology, relapse of the tumor in atrophic scars, localization of BCC in the area of the underlying cartilaginous tissue, bleeding of the tumor). Nowadays an electron beam, close-focus X-ray therapy, low-voltage or orthovoltage X-ray therapy, gamma and electron therapy, radium needle implantation are used as a part of radiation therapy.13,14,18 Recurrence in this method is 7.5% in primary BCC and increases with the localization of sites in anatomically difficult areas to 30%.18 Radiation therapy often causes a number of complications (radiation dermatitis, perichondritis, etc.) leading to serious cosmetic defects requiring a long period of treatment and rehabilitation.9,18 A modern approach to the treatment of superficial forms of BCC in the presence of contraindications to the use of other methods is pathogenetically substantiated local or systemic drug-induced effects on the process of carcinogenesis (interferon-alpha, interleukin-2, imiquimod).2,18 In recent years, interstitial interferon (IFN) therapy has been increasingly used in this country and abroad for the treatment of BCC, which is associated with a marked immunomodulating and antitumor effect. The most widely used is recombinant interferon alpha-2 (Intron A, Reaferon), which is associated with its ability to suppress the expression of oncogenes and the production of tumor growth factors by a significant antiproliferative effect. IFN-a2 is able to increase the activity of natural killer cells, macrophages, T-lymphocytes.5,13,16,20 In the presence of T2-T3 tumors and 2-3 degree of ulceration, the size of the tumor decreases, which makes it possible, if necessary, to apply aggressive methods of treatment in a smaller volume.20 The clinical effect is characterized by painless tumor resolution during the follow-up period. Puncture can be used in all forms of BCC, regardless of the tumor site and size. The number of courses can vary from 1 to 4, which leads to a complete regression of the tumor or a significant (more than 50%) decrease in its size, depth, ulcerous tumor destruction. After the treatment, stable positive dynamics of the interferon-α and -γ (INF-α m IFN-γ) levels in blood serum is observed, which eliminates the interferon deficiency (I-II degree), found in all patients before the treatment.3,4 Here are some of our clinical examples of successful treatment of complicated localization cases of basal cell carcinoma by the method of complex intralesional interferon-α therapy (Figure 1)(Figure 2).
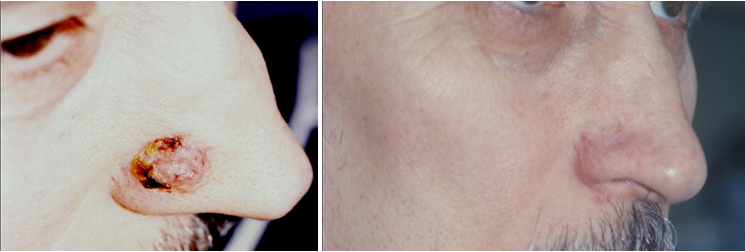

Interferon therapy can be recommended to the patients for whom other types of treatment are not possible, with inoperable or large basalomas, dangerous and cosmetically risky.21 Carbon dioxide, neodymium, argon laser therapy for BCC are widely used.9,18 Laser treatment is used for the purpose of photothermal destruction of the tumor (coagulation, excision), as well as of photodynamic therapy, which is based on the induced sensitization of tumor tissue to light.13 Indications for laser coagulation: small (up to 2 cm in diameter) tumors, primary multiple sites of small size, including localized in difficult accessible areas for other methods of treatment (external auditory canal, wings of the nose, ear auricle, etc.).13,16 The advantages of this method are gentle effect, simultaneous removal of the tumor with coagulation of blood vessels. To obtain a more significant antitumor effect, laser therapy should be combined with various cytostatic drugs and radiotherapy.13,14,16,20 The recurrence rate of BCC after laser therapy is from 0.8 to 4%.2,9,11,19 PDT using various photosensitisers (photophryn, photolon, methyl-aminolevulinic acid, etc.) is an effective and non-invasive method of BCC treatment. Photosensitisers are able to selectively accumulate in tumor cells under the influence of laser radiation in the presence of oxygen, as a result of interaction with proactive porphyrins, nontoxic triplet oxygen goes into a more active state, forming "singlet" oxygen and other high-activity radicals, leading to selective death of malignant tumor cells and its vascular stroma. At the same time, normal tissues surrounding the tumor are damaged insignificantly, due to low accumulation of photoactive porphyrins.22‒24 Indications for use are superficial, micronodular, multiple forms of BCC with the size not exceeding T1-2. Relapses are observed in 14.2% of cases.7,9 The use of local chemotherapy with cytostatic creams (5% 5-fluorouracil, 5-10% phthorafurum, 30-50% prospidinum, 5% omain, 5% glyciphon) is recommended for elderly weak patients who cannot tolerate more aggressive types of treatment and has a palliative effect.9 Electrochemotherapy is the process of delivery of a chemotherapeutic drug to the tumor (5-fluorouracil, bleomycin, doxorubicin, vinorelbine, etc.) under the influence of galvanic current. The galvanic current used enhances the antitumor effect of drugs, and it has an antitumor effect itself. As a result of electrochemotherapy, a complete resorption (destruction) of the tumor was obtained in 69.3% of cases, a partial resorption of the tumor – 19.8%, stabilization – 9.9%, a progression – 1% of patients. The best results of treatment were obtained in patients with superficial forms of tumors, where a complete resorption was obtained in 97.7% of cases.25
An innovative and promising approach to BCC therapy, in particular of severe and inoperable forms, is the use of drugs that inhibit the pathogenetic pathways of tumor development, in particular, the mechanism of pathological activation of the Hedgehog signaling pathway, which occurs in approximately 90% of patients with BCC. The Hedgehog signaling pathway plays an important role in the process of growth and formation of organs and tissues during fetal life. It has been shown that the pathological activation of the signaling pathway in an adult increases the proliferation of cells and causes their resistance to the apoptosis process.2,25 Vismodegib and Sonidegib selectively block the signal transmission along the The Hedgehog pathway, specifically inhibiting its key pathologically active signaling protein SMO, thus preventing the expression of genes that stimulate tumor growth.2,26 As a result of the tests, the most frequent side effects were alopecia, muscle spasms, nausea, taste disorders, fatigue and increased CK levels in blood.26 In recent years there has been a tendency to use combined methods of BCC therapy, which allow increasing the treatment effectiveness. The use of the complex therapeutic strategy provides a significant influence on the immune parameters, which improves the general state of patients and reduces the risk of tumor recurrence.2,5,6 Vismodegib and photodynamic therapy is considered to be a promising combination therapy. The effectiveness of this combination was confirmed by an open study conducted in the United States, for which patients with multiple sites of BCC were selected. During 3 months, patients took Vismodegib 150 mg and had 3 courses of PDT. The results were assessed after each procedure and 30 days after the study. The study concluded that combination therapy exceeds each of these treatment methods used separately in effectiveness and reduces their toxicity, reducing the risk of side effects of each of them. After the therapy, 90% of the sites were completely regressed and 10% - partially, recurrence was observed in 1% of cases.27 The newest innovation in the sphere of combination BCC therapy, which is in Phase II of clinical research, is the combination of inhibitor of atypical protein kinase C (aPKC) and histone deacetylase 1 (HDAC1). Progressive BCC often acquires resistance to SMO inhibitors through unknown mechanisms. SMO mutations are defined in 50% (22 of 44) of cases of progressive BCC. These mutations support signaling along the Hedgehog pathway even in the presence of SMO inhibitors (Vismodegib and Sonidigib), through the GLI 1 transcription activator, to withstand the high level of signaling necessary for cell survival.28 In computer simulation of the experiment, a drug was developed that inhibits the mutation and expression of genes, the drug was recognized and approved by the FDA as an inhibitor of histone deacetylase 1 (trade name is Vorinostat). The study has proved that Vorinostat stops cell division in BCC in high doses, but the necessity of using high doses restricts its use as monotherapy, because of the risk of a large number of side effects. In the further study, atypical protein kinase C was detected, the molecule of which is the histone deacetylase 1 costimulator in signaling along the Hedgehog pathway, linking two loops of up regulation. The drug called Parthenolide was offered to inhibit its effects. This drug was approved by the FDA, and its combination with Vorinostat can minimize side effects and significantly reduce the dose necessary to achieve the result both in vitro and in vivo in patients with BCC, as the therapeutic panel expands.29
Therefore, achievements in the biology of BCC have led us to understand the damage development ways and prompted clinicians to require accuracy and reliability in the morphological classification of basal cell carcinoma in order to optimize therapeutic routes.
The study had no sponsorship
The authors declare no conflict of interest.

©2018 Snarskaya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.