MOJ
eISSN: 2379-6294


Research Article Volume 3 Issue 7
Department of Botany, Guru Ghasidas Central University, India
Correspondence: AK Dixit, Department of Botany, Guru Ghasidas Central University, Bilaspur 495009, Chhattisgarh, India, Tel 9175 8719 5600
Received: September 15, 2017 | Published: November 30, 2017
Citation: Bhaskar M, Dixit AK. Toxicity evaluation of hasdeo river water on seed germination and amylase activity in vigna radiata l. MOJ Toxicol. 2017;3(7):161–165. DOI: 10.15406/mojt.2017.03.00075
The present study is focused on chemical characterization and the toxicological aspects of Hasdeo River water as the study area which is profoundly affected by domestic and industrial activities. Studies revealed that pollution stack in Hasdeo River was surpassed many folds from the desirable limit in downstream sampling (PP, HP1 and HM1) sites compared to upstream sites. The tested metal like Cr was found higher in all most all the sampling sites from the desirable limit of BIS, whereas Cd was below detectable level or within a desirable limit. Toxicological evaluation on Vigna radiata L. illustrated that the relative seed germination was 100% in all the sampling sites. Some sites (K1, K0, K2 and HP2) showed enhanced root and shoot growth, germination index and amylase activity as compared to control but some sites (PP and HP1) showed reducing effect. This pattern concluded that organic pollution at certain limit enhances the plant growth. Finally on the basis of chemical texture of river water and toxicity evaluation we conclude that the quality of Hasdeo River was compromised at many points. It is urgent need to take proper actions to check the pollution load in Hasdeo River.
Keywords: hasdeo river, chemical texture, toxicity evaluation, germination index, amylase activity
APHA, american public health association; ANOVA, analysis of variance; BIS, bureau of indian standard; BOD, biological oxygen demand; CD, cadmium; COD, chemical oxygen demand; Cr, chromium; GI, germination index; ICPMS, inductive coupled plasma mass spectrophotometer; WHO, world health organization; IU, international unit
River water is a very important natural resource of water for meeting the requirements of various sectors in the world and for this reason cannot be looked upon in isolation.1 The Hasdeo River is second largest tributary of the Mahanadi River system in East Central India. Hasdeo River in Chhattisgarh is influenced by the various problems imparted by excessive industrialization, urbanization and rapid agricultural usage similar to other riverine system. The water quality of Hasdeo River has been deteriorating due to loss of their self- purification capacity with the addition of metal and organic pollutants day by day.2 This pollution not only affects water quality, but also directly or indirectly threats human health, economic growth and social prosperity. In developing countries like India mostly rivers are polluted. In India, mostly farmers irrigate their crops with river water having contaminants like high amount of organic compounds and heavy metals due to unavailability of alternate sources of irrigation that ultimately affects biochemical response of crop plants and yield.3,4 The evaluation of metal toxicity and organic pollutants present in the river water are immensely important to determine their phytotoxicity, before their possible utilization in agriculture and human consumptions. On other hand, concern about the adverse effect of polluted Hasdeo River water has not arises until the last decade. Hence, this study deals with the toxicological evaluation of Hasdeo River water by using seeds of Vigna radiata L. in terms of seed germination, seedling growth and amylase activity for environmental safety. This study was extended to analyze chemical texture of Hasdeo River water for validation of this toxicological behavior.
Study area and sampling
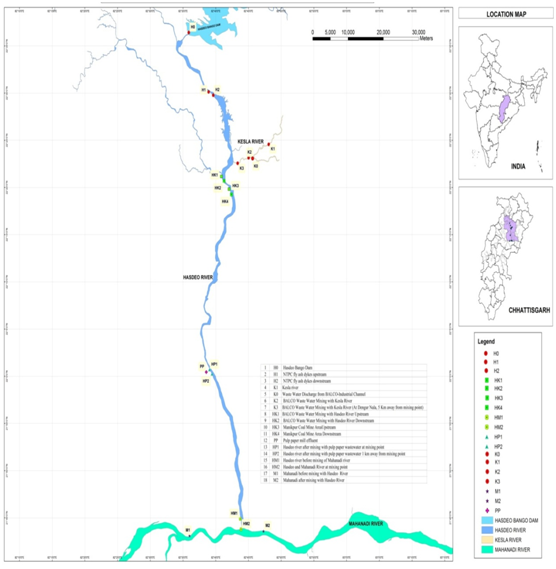
In this study, 18 sampling sites have been selected for Hasdeo River water to assess industrial and domestic impact. The sampling sites, localization of the study area of Hasdeo River are summarized in Figure 1. All the sample collection was done in August 2015. The samples were taken into pre-sterilized container (10 liter capacity), and transported to the laboratory as soon as possible. All the sampling processes were done as described in standard method.5
Analytical procedures
pH (Orion ion meter Model-960) and electrical conductivity (EC) (Thermo Orion Model-162A, USA) were determined without filtering the water sample. Phosphate and sulfate was measured by vanadomolybdo-phosphoric acid colorimetric and BaCl2 precipitation methods, respectively. Total Hardness (T- hard as CaCO3), color and COD were determined by the EDTA titrimetric method, visual comparison method and open reflux method, respectively. Prior to total elemental analysis, water samples were digested with digestion mixture of nitric/ perchloric acid (5:1). The concentration of heavy metals like Cadmium and Chromium were measured using inductively coupled plasma spectrophotometer (Thermo Electron; Model IRIS Intrepid II XDL, USA). The physicochemical parameters of river water were analyzed by using the standard protocols of APHA.5
Toxicity evaluation
By seed germination test: For seed germination test, collected Hasdeo River water from each 18 sites was used (v/v). All the seeds were surface sterilized by the help of 2 % HgCl2 to avoid any fungal contamination. Afterward, ten seeds of Vigna radiata L. were bedded with what man filter paper in presterilized Petri dish of uniform sized. These filter papers were then moistened with 5 ml of tap water for control and with same volume of different river water samples. The plates were incubated at 30°C in BOD incubator for 6 consecutive days. All experiment was performed in triplicate fashion. The criterion of germination which we have taken was the visible projection of radical from seed coat and it express in terms of percentage. The germination index (GI) was calculated by the formula of Gomare et al.6
By amylase assay: Twenty seeds of each treatment were selected and homogenized with 0.1 M acetate buffer (pH4.7), carefully filtered with two layers of cheese cloth. This filtrate was centrifuged at 15000 rpm at 4°C and supernatant was used as crude enzyme for α amylase activity. For α amylase assay, reaction medium (4.5ml) contain 1.5ml of acetate buffer (pH 4.7), 0.5ml crude enzyme diluted to 1.5ml using same buffer and 1.5ml of 0.1 % starch solution. The reaction medium was incubated for 15 minute at 30°C and then reaction terminated by adding 1 ml of 0.1% iodine solution and 4.5 ml of 0.05NHCl. The absorbance was measured in visible spectrum at 620nm and reduction in absorbance was express in amylase activity.7
Statistical analysis
For continuous data, normality was tested using Kolmogorov Smirov test. Categorical data were presented in mean and standard deviation. One way ANOVA was used for physicochemical parameters to make comparison between more than two mean followed by Tukey test. Statistical analysis and graph construction were carried out using the statistical package for the social science, version 22 (SPSS-22, IBM, Chicago, USA).
Approximately 125 km area was covered under this investigation, with 18 sampling sites starting from Hasdeo Bango dam to the point where Hasdeo River meets with the Mahanadi River (Hasdeo Bango Dam 22°36’24.8N 82°35’52.8E to Mahanadi River downstream 24°43’47.3N 82°43’51.9E (Figure 1). From origin point to end point its water is being utilized for irrigation through various dams and barrages and for industrial and domestic usages. In recent two decades catchment area of Hasdeo River are heavily industrialized especially Korba and Champa district. Wastewaters from different Industrial area having different type of organic and inorganic pollutants are collected and finally discharge to next door river system without any proper treatment.
The chemical texture of hasdeo river water
The range of pH values was 6.94±0.35 to 8.08±0.12 (mean: 7.51) (Table 1). These values were within the standard limit (pH: 6.5 to 8.5) set by the Bureau of Indian standard (BIS).8 Analysis of variance (ANOVA) revealed that most of the sites had almost same pH and showed homogenous nature (ANOVA/Tukey test; p > 0.05). Conductivity varied widely from 22±0.2 to 3053±33 µS/cm (Table 1). Almost 50% river water samples significantly exceeded the desirable limit of conductivity that is 1000 µS/cm(ANOVA, Tukey p < 0.05). The difference was least significant among some river water samples, collected from site H0, H1, H2, K1, K0 (Tukey p > 0.05). Highest color 206.47±10 CoPt (Cobalt Platinum Color Unit) was observed at site PP might be due to higher accumulation of different organic and inorganic compounds coming from different industries of Korba and Janjgir region. The organic pollution parameter COD adversely affect river water quality and biodiversity.9 Maximum elevated COD was found at site PP (237±33mg/L) followed by HP1 (183±22mg/L) (Tukey test; p< 0.05). Elevated levels of COD indicated poor water quality at these sites might be caused by sewage, urban, agricultural and industrial effluents. Pulp paper effluents contribute to higher COD values in water bodies10 it is consistent with our result as both sites PP and HP1 were impacted with pulp paper mill effluent. The sulphate was within the limit of drinking water standards of WHO11 that is 250mg/L (Table 1). The phosphate was highest at sampling site HP1 (1.39±0.15mg/L). Water hardness is caused primarily due to the polyvalent ions (mainly calcium and magnesium). Total hardness was ranged from 50 mg/L to 546.7±12mg/L at all the sampling sites. Only one site PP has exceeded the prescribed limit of total hardness (500 mg/L) and the difference was significant to other sampling sites (Tukeyp<0.05). In this study cadmium (Cd) was below detectable level or within a desirable limit (0.01mg/L) except HP2, HM1, HM2 and M2. Chromium (Cr) was higher in all most all the sampling sites from the desirable limit (0.05mg/L) approved by BIS,8 except site H0, HK3, and M1. Sources of Cr in Hasdeo River could be discarded chromium batteries, surface runoffs, and solid waste dump leachates.12
Sampling Sites |
pH |
Conductivity (µS/cm) |
Color (Copt.) |
COD |
SO4-2 |
PO4-3 |
TH |
Cd |
Cr |
H0 |
7.2±0.3 a |
22±0.2a |
BDL |
3±1 |
BDL |
BDL |
BDL |
ND |
0.012±0.007a |
H1 |
7.95±0.3 b |
340±17.8a |
65.11±4a |
17±1a |
46.08±3a |
0.25±0.02a |
94±5.6a |
ND |
0.69±0.04 |
H2 |
7.88±0.22 b |
557±21.3a |
169±13.5 |
22±1.5ab |
17.84±1 |
0.27±0.02a |
123±8.6b |
ND |
0.57±0.08 |
HK1 |
7.65±0.2 b |
970±39a |
73.64±2.8b |
30±2.9b |
43.02±1.7a |
0.45±0.09 |
146±4.7 |
0.01±0.01 |
0.102±0.03 c |
HK2 |
7.48±0.3 b |
1815±68cb |
72.1±3.2b |
80±3 |
104±4.6 |
0.7±0.09 |
470±23 |
0.011±0.01 |
0.054±0.01d |
K1 |
8.08±0.12 b |
299.7±17.1a |
81.22±4.8d |
15±1.7ab |
44.42±3.2a |
0.28±0.01ab |
50±1.1 |
0.01±0.01 |
0.18± 0.08b |
K0 |
8.0±0.2 b |
712±31a |
81.39±3.6d |
39±2.5 |
31.6±1.5 |
0.34±0.2b |
164±3.6 |
ND |
0.17±0.02b |
K2 |
7.99±0.2 b |
606±26a |
112.4±9.3c |
33±1.3b |
44.56±3a |
0.25±0.06ac |
124±4.1b |
ND |
0.113±0.03c |
K3 |
7.92±0.5 b |
511.5±20a |
81.36±3.9d |
19±1.3ab |
62.16±1.8b |
0.34±0.04b |
82±2.3a |
ND |
0.11±0.02c |
HK3 |
7.49±0.2b |
1175±56b |
65.11±3.8a |
60±4.8c |
70.26±4.9c |
0.2±0.1ac |
266±14 |
ND |
0.04±0.01d |
HK4 |
7.39±0.2a |
1400±30b |
115.1±9.2e |
68±4.7c |
66.34±4.6bc |
0.18±0.01c |
356±25 |
ND |
0.054±0.01d |
PP |
8.03±0.2b |
3053±33c |
206.47±10 |
237±33 |
166±13.75 |
BDL |
546.7±12 |
0.001±0.001 |
0.19±0.02b |
HP1 |
7.5±0.13b |
2931±34c |
103.92±0.4 |
183±22 |
121±57 |
1.39±0.15 |
444±17 |
ND |
0.151±0.07 |
HP2 |
7.3±0.2a |
2173±5.5cb |
88.29±49 |
147.91±25 |
96.84±2.1d |
0.85±0.4d |
428±16c |
0.05±0.006 |
0.12±0.078c |
HM1 |
7.2±0.9a |
2094±89cb |
80.46±19d |
135±82d |
90.56±73d |
0.8±0.15d |
425.5±43cd |
0.04±0.037 |
0.12±0.006c |
HM2 |
7.3±0.5a |
2043±46cb |
76.92±4bd |
132±31d |
83±34 |
BDL |
414.6±12de |
0.05±0.003 |
0.12±0.009c |
M1 |
6.94±0.35 |
700.5±35ab |
13.46±68 |
70±5c |
7.64±0.38 |
0.80±0.1d |
1.65±0.62 |
ND |
0.02±0.01a |
M2 |
7.03±0.8a |
1550.4±71c |
50.63±22 |
108±35 |
71.26±4.56c |
BDL |
406±0.83e |
0.05±0.04 |
0.11±0.01 c |
Table 1 The chemical texture of Hasdeo river water
All the parameters are in mg/L except temperature, conductivity, color and pH. BDL: Bellow Detection Limit; Different alphabets show significant variation. (ANOVA, Tuky p=<0.05)
Evaluation of river water toxicity
In India, small or medium scale industries still have conventional treatment technologies, but these treatment technologies are not efficient to treat the wastewater released by different industries. The resent treatment techniques like physical and chemical treatments (ozonation, carbon nanotreatment etc.) are very expensive and need high installation and operational cost, hence, the small or medium scale industries dispose treated or partially treated effluent to the next door river system. Therefore the quality of river water was compromised. This river water with high pollution parameters like high BOD, COD, metals etc. are used by farmers for irrigation of agriculture field which are deleterious for environmental health.4,9 Moreover, plant growth parameters were also affected due to high concentration of potentially toxic metals, high COD values and ions (SO42−, PO43−, K+ and Cl−) which may present in polluted river water.13
The range of radical length was observed from 3.14 to 5cm for all sampling sites (Table 2). Almost 77.8% samples had shorter radical’s length as compared to tap water control. However, seeds treated with river water collected from different sites had longer plumule length as compare to tap water control. The range of plumule length was 8.23-16.3 cm for all sampling sites. The relative seed germination was found 100% in all the sampling sites. Water samples collected from different sites, namely K1, K0, K2 and HP2 had supported the root, shoot growth, GI and their values were relatively higher than tap water control. On the other hand, some reducing effect was observed in seeds treated with water samples collected from site PP and HP1. These findings are in accordance with the observation of Bhargava and Chandra13 who reported that the promotion of plant growth parameters of certain pollution load might be due to presence of optimum level of plant nutrients which are present in the form of nitrogen or other minerals in water. But, at elevated pollution loaded sites like PP and HP1 has deleterious effect on seedling growth parameters showing reduction in root, shoot length and GI. The negative effect of water sample collected from PP followed by HM1, HP1 sites may be due to higher concentration of heavy metals, organic load and different ions, which retarded the seedling growth by affecting the water absorption and other metabolic activity.13-15 Seed germination is a complex process in plants that can be affected by a range of environmental factors. Starch is the major constituent of the world’s crop yield and degradation of starch is very essential for seed germination. In germinating seeds, starch degradation is initiated by an enzyme called amylase producing soluble oligosaccharide from starch.16,17 Starch hydrolyzed by amylase to release maltose and finally alpha glucosidase bond breaks down maltose into glucose and providing energy to germinating seeds. In this study, the optimum enzyme activity was recorded in seeds treated with river water collected from K0, K1, HP2 and K2 (range 0.64-0.61 IU) (Figure 2).
Sampling Sites |
RL Mean (cm) |
PL Mean(cm) |
Germination (No of Seeds after 24 hrs) |
Relative Seed Germination (%) |
Relative Root Elongation (%) |
GI (%) |
Control |
4.78 |
8.82 |
10 |
100 |
100 |
100 |
H0 |
4.13 |
8.23 |
10 |
100 |
86.4 |
86.4 |
H1 |
3.92 |
12.5 |
10 |
100 |
82.01 |
82.01 |
H2 |
4.67 |
12.91 |
10 |
100 |
97.7 |
97.7 |
HK1 |
4.55 |
16 |
10 |
100 |
95.19 |
95.19 |
HK2 |
4.65 |
16.36 |
10 |
100 |
97.28 |
97.28 |
HK3 |
4.7 |
15.48 |
10 |
100 |
98.33 |
98.33 |
HK4 |
4.42 |
13.59 |
10 |
100 |
92.47 |
92.47 |
K1 |
4.9 |
12.23 |
10 |
100 |
102.51 |
102.51 |
K0 |
5 |
12.44 |
10 |
100 |
104.6 |
104.6 |
K2 |
4.86 |
15.49 |
10 |
100 |
101.67 |
101.67 |
K3 |
3.86 |
13.84 |
10 |
100 |
80.75 |
80.75 |
PP |
3.14 |
9.81 |
10 |
100 |
65.69 |
65.69 |
HP1 |
3.8 |
10.2 |
10 |
100 |
79.5 |
79.5 |
HP2 |
4.92 |
11.18 |
10 |
100 |
102.93 |
102.93 |
HM1 |
3.67 |
10.73 |
10 |
100 |
76.78 |
76.78 |
HM2 |
4.19 |
12.03 |
10 |
100 |
87.66 |
87.66 |
M1 |
4.43 |
14.24 |
10 |
100 |
92.68 |
92.68 |
M2 |
4.45 |
9.71 |
10 |
100 |
93.1 |
93.1 |
Table 2 Toxicity evaluation of Hasdeo river water sample on Vigna radiate L
RL: Radical length; PL: Plumule length; GI: Germinating Index
The enzyme activity of control (tap water) was > 0.54 IU (International Unit). However, the seeds treated with river water collected from site PP and HP1 showed the minimum amylase activity (range 0.3-0.33 IU) indicating that some sites of Hasdeo River are seriously affected by industrial activity. The reduction in amylase activity might be due to the high salt load and metals content affecting various physiological and biochemical process of seed germination. These results were consistent with other researchers.4,13,18
In this study, toxicological evaluation of Hasdeo River water has been carried out along with the chemical texture. In totality the quality of Hasdeo River water is compromised due to industrial activities mainly at downstream river sites like PP, HP1 and HM1. If environmental agencies do not focus on the current status of Hasdeo River, the situation will be alarming in next decade and it will affect human directly or indirectly.
The authors are grateful to the financial assistance from Rajiv Gandhi National Fellowship (RGNF) by University Grand Commission (UGC), it is highly acknowledged.
The author declares no conflict of interest.

©2017 Bhaskar, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.