MOJ
eISSN: 2379-6294


Review Article Volume 1 Issue 3
Department of Chemical Engineering, University of Waterloo, Canada
Correspondence: Sylviane Pulvin, Department of Chemical Engineering, University of Waterloo 200 University Avenue West, Waterloo, ON N2L 3G1, Canada, Tel 5198884567
Received: April 15, 2014 | Published: June 12, 2015
Citation: Tanvir S, Pulvin S, Anderson WA. Toxicity associated with the photo catalytic and photo stable forms of titanium dioxide nanoparticles used in sunscreen. MOJ Toxicol. 2015;1(3):78–94. DOI: 10.15406/mojt.2015.01.00011
The increasing number of applications using titanium dioxide nanoparticles (nano-TiO2) highlights the need to continuously and systematically investigate its toxicity. Particle size, surface area and dose are the classical parameters considered when performing toxicity studies. However, consideration of the size-related properties and altered reactivity can unveil complex and unexpected phenomena arising from the interplay of the different factors. In addition to particle size, altered reactivity can be induced by intentional or unintentional modifications of the nanoparticles by their surrounding matrix. This effect could potentially influence the nanoparticles’ band gap, surface reactivity, agglomeration, mobility and photo catalytic behavior. The remarkable ability to absorb impurities from the surrounding medium could transform nano-TiO2 into a surrogate carrier of trace elements (e.g., heavy metal ions), which heightens their transportation and intracellular accumulation. This review outlines the different characteristics and interactions that may contribute to the underlying mechanisms of health and environmental toxicity of nano-TiO2, and identifies gaps in current understanding.
Keywords: nanomaterials, nanotoxicology, nano-tio2 toxicity, titanium dioxide toxicity, sunscreens, cosmetics
IARC, international agency for research on cancer; UV, ultraviolet; QD, quantum dot; ROS, reactive oxygen species; PBS, phosphate buffered saline; CB, conduction band
The properties of materials change as their size approaches the nano particle scale, which is generally considered to be that with at least one dimension of 100nm or less. The toxicity of nanoparticles has been linked to such characteristics as elemental composition, charge, shape, Crystallinity, surface area, solubility and surface chemistry/derivatization.1–6 Understanding the nano particle-cell interaction is thought to be critical for the safe development of nano materials.7 It seems unlikely that the potential toxicity of nanoparticles and the underlying mechanisms can be predicted or explained by any single unifying concept.
This review is focused on Titania (TiO2) for which there is substantial interest in the chemical, biological, and industrial worlds because of its fascinating and useful physicochemical properties. Currently, information describing the relative health and environmental risks associated with nano-TiO2 is severely lacking. Only recently, critical questions regarding the potential human health and environmental impact of nano-TiO2 have been raised.8–11 TiO2 particles have long been considered to pose little risk to respiratory health because they are both chemically and thermally stable.12 However, TiO2 is classified as a Group 2B carcinogen by the International Agency for Research on Cancer (IARC) based on the findings of lung tumor induction in female rats.13,14
Nano-TiO2 is attractive for use in a large number of applications based on its unique optical and photo catalytic properties, tunable band gap, thermal stability, chemical resistance and hardness.15 Because of the relatively large band-gap, the particles absorb the higher-energy (shorter wavelength) ultraviolet radiation making it a useful constituent in sunscreen products. TiO2 photo catalysis is widely used in the fields of wastewater treatment,16 sterilization,17 self-cleaning,18 hydrogen evolution,19 and photoelectron chemical conversion.20 Normally, TiO2 can only be excited with ultraviolet (UV) light because of its wide band gap,21 although this is considered a drawback when photo catalytic conversion is desired under visible light. The crystalline structure is the major factor determining the band gap, but secondary factors include the particle size and the presence of defects (physical or chemical).22,23 A large surface-to-mass ratio of the nanoparticles helps to promote catalytic reactions, and increases their ability to absorb and carry other compounds. Their surface reactivity originates from quantum phenomena that can make nano-TiO2 seemingly unpredictable.24
Engineered nano-TiO2 is designed to impart specific characteristics that vary according to their use. Aside from unique nanoscale properties (size, Crystallinity, reactivity, and thermodynamics), nano-TiO2 may be functionalized,25 doped26 and coated to control photo catalytic activity. The differences between the applications of nano-TiO2 in water remediation technologies versus consumer products are important, but the currently available information does not allow the suited differentiation. The purpose of this review is to highlight the informational gaps and to describe the physical and chemical features that may be important when performing nano toxicological studies on nano-TiO2.
Nano-TiO2 in remediation technologies versus sunscreen
Photo catalytic nano-TiO2 is in demand for remediation, sterilization and sanitation, whereas photos table nano-TiO2 is recommended for sunscreen use. A way to enhance or attenuate nano-TiO2 photo activity is to manipulate the surface chemistry. Anatase is a particularly photoactive form of TiO2 and is used for photo oxidation reactions.27 The development of photo catalysts exhibiting high reactivity under visible light allows for a greater fraction of the solar spectrum to be used, which would be an advantage in both water and air treatment technologies. The visible light-activated TiO2 can be prepared by dye sensitization,28 external surface modifications,29,30 or band gap tailoring by doping.26,31
Nano-TiO2 acts as a sunscreen in two ways, namely absorption and scattering, which are dependent on the light wavelength. In the past, sunscreens containing metal-oxide particles appeared as opaque, white topical creams that were considered to be “unattractive”. However, more transparent preparations are now available from a number of manufacturers, which has led to the use of nanoparticles in cosmetic formulations. Metal oxide particles used in such formulations span a wide range of sizes, shapes, and surface coatings. Because of the high reactivity of anatase, the rutile form of TiO2 is preferred for sunscreen use to minimize the photo catalytic effects,32 although some sunscreens still contain photo reactive nano-TiO2. TiO2 particles are typically formulated in skin lotions as oil/water emulsions. Concerns have been raised about the product labeling issues33 and the role of the regulatory authorities in the process of identifying environmental health and safety risks related to nano-TiO2 modifications.11,34 Manufacturing technologies can produce nanometer-sized particles, but once they are formulated into a lotion agglomeration and aggregation may occur. Coatings and surfactants are used to aid in the dispersal of metal oxides; however, measuring particle size in the formulation is difficult using standard methods.35 This is particularly true after application to the skin, where surface pH, salts, and oil may affect the coatings’ dispersion and size. These issues are rarely addressed in the literature.
From conventional to nano
The term “conventional” is used to make an explicit distinction between the nanoscale material and other forms of TiO2 not having the special characteristics of nano-TiO2. Nanoparticles have a high surface are a per unit mass resulting in a high excess free energy per unit mass. This may partially explain why unique properties can be observed at the nanoscale level that are not present in the same coarse material, such as the size-dependent fluorescence emission frequency of semiconductor quantum dot (QD) nano materials. These are characterized by a high excess of energy at their surface and are thermodynamically unstable.3,4,36–38 Crystallographic changes, such as lattice contraction or deformation, the appearance of defects, or rearrangements of the surface atoms or changes in morphology may occur to stabilize the nanoparticles. These unique nanoscale features affect the interfacial reactivity and the intrinsic properties of the nanoparticles. Research programs need to focus on size-related properties rather than on size alone to evaluate the safety of engineered inorganic nanoparticles. Ignoring the differences between small and truly “nano” particles may lead to inadequate interpretations of experimental results.38
Physicochemical properties of Nano-TiO2
The physicochemical properties of nano materials that have been identified as important factors in uptake and toxicity include crystal structure, size, surface charge, surface energy, and chemical composition. For human health and environmental risk evaluation, consideration of multiple aspects is required.
Crystal structure
TiO2 has three main crystalline structures: anatase (tetragonal), brookite (orthorhombic), and rutile (tetragonal). Different structures lead to different physical properties, which leads to their usage in a variety of applications. For example, anatase is employed for photo catalysis because of its high photo reactivity, while rutile’s good light scattering makes it useful for pigments.39 Because anatase is more photoactive than rutile, free radical formation potential is likely higher for anatase. Regardless of the crystal form, nano-TiO2 is used in commercial sunscreen formulation. Furthermore, an anatase-rutile mixture is more effective than each phase separately in photo catalysis, which is dependent on the electron-hole recombination rate, crystallinity, adsorptive affinity and particle interconnection.40
Early nano toxicity studies have yielded conflicting data identifying either the size or the crystal structure as the mediating property for nano-TiO2 toxicity.41–43 Some recent studies44–47 emphasize the contribution of the crystalline structure of nano-TiO2 to the toxicity. In these studies, anatase TiO2 was found to be more potent than the rutile form of the material. Jiang et al.46 used a cell-free assay to determine the ability of nanoparticles to generate reactive oxygen species (ROS), finding a significant dependence between particle size and capacity to generate ROS. There was a clear transition in behaviour with anatase nanoparticles between ~10 and 40nm, with the smallest particles demonstrating a reduced capacity to generate ROS. Since it is known that the surface structure of materials can change at very small sizes,48 it is still uncertain whether this transition was size or surface chemistry mediated. The findings might be related to the density of defects on the surface of the particles, which could be another physicochemical parameter of interest in understanding the toxicity of nano materials, as suggested by the authors.
Size
The size of the particles is generally used to define materials as “nano-sized.” This definition appears straight forward; however it is subject to several difficulties. First, discrepancies can arise due to the different methods and calculations used to measure particle size. Size measurements are usually based on a distribution of nanoparticles and the estimated size can be averaged by volume, weight, or area. The measurement devices use a specific environment, such as in hydrodynamic or aerodynamic sizing, and they require pre-treatment methods before measurement that can lead to discrepancies with the actual size of the nanoparticles (as determined by imaging techniques) in some circumstances.
The size, surface charge and morphology of nanoparticles exert a significant influence on the physical and chemical properties that influence their interactions with biological systems. For example, the hydrodynamic size and surface charge of nano particle dispersions can have an effect on the way in which an organism responds to exposure. The above issues have not been properly characterized in the past. This influence includes absorption, distribution, metabolism, and excretion.49–51 The size dependency of TiO2 toxicity has been demonstrated,41,52–55 appears to be applicable to a variety of TiO2 forms, and occurs regardless of the experimental settings. However, the results are based on a limited number of studies that focused on TiO2 and carbon black particles, and have fostered the perception that all nanoparticles are likely to be more toxic than larger-sized particulates.42,56 However, additional factors such as differences in the TiO2 form, particle aggregation/disaggregation potential, surface coatings and/or surface charge and the method of particle synthesis (i.e. whether the particle was generated in the gas or liquid phase) may be important variables influencing toxicity.57,58 Furthermore, a high degree of particle aggregation is associated with TiO2 administration such that exposure to mono disperse particles is unlikely to occur.59
Agglomeration
The terms “agglomeration” and “aggregation” are often used interchangeably in the field of nano toxicology. However, some authors have suggested that nano particle aggregation and agglomeration are distinct phenomena, whereby agglomerates are formed by clusters of particles that are held together by electrostatic interactions, while aggregates are formed by covalently fused or sintered particles that are not easily separated.60 TiO2 nanoparticles have a large specific surface area but can easily form agglomerates in suspension depending on the strength of the particle-particle and particle-media interactions.61 Important parameters governing the state and stability of nano particle dispersions in water (Figure 1) include solution ionic strength, pH, surface charge (zeta potential), and surface coating (hydrophobic/hydrophilic). The degree of agglomeration is determined by the magnitude of the zeta potential in aqueous media.When nanoparticles are dispersed in an aqueous medium, surface ionization and the adsorption of cations or anions results in the generation of surface charge, resulting in the development of an electric potential between the particle surface and the medium.When the zeta potential is close to zero (isoelectric point), particles tend to agglomerate, butat highly negative (high pH) or positive (low pH) zeta potentials, particles in dispersions tend to repel each other such that no agglomeration occurs.
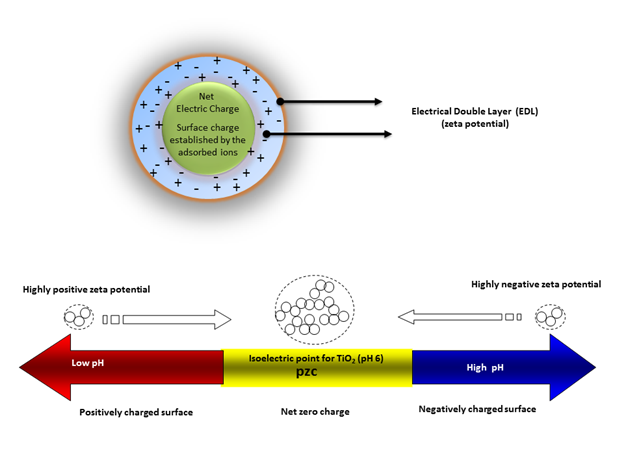
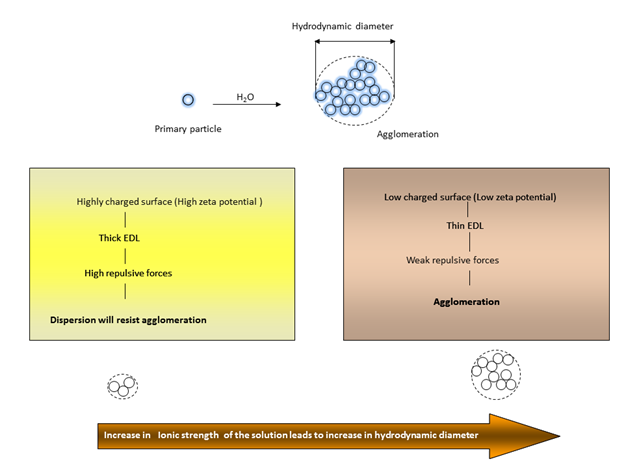
Important factors that affect zeta potential include pH, ionic strength, and additive concentration. Ionic strength influences the nano particle’s dispersion stability by changing the electrical double-layer around the particle. The electrostatic double-layer (Figure 1) (Figure 2) decreases with increasing ionic strength and consequently, weak repulsive forces result in agglomeration with large hydrodynamic diameters. At any given pH, an increase in ionic strength generally results in increased agglomeration (Figure 2). An increase of the ionic strength diminishes the magnitude of the electrostatic repulsion, thereby resulting in an intensification of the agglomeration phenomena. Particle agglomeration is minimized by increasing surface charge, due to enhanced electrostatic repulsions between nanoparticles. The surface charge is controlled by several mechanisms, including surface ionization, ion adsorption, and lattice ion dissolution. Generally, particles have a positive surface charge at a low pH and a negative surface charge at a high pH.
Toxicity studies have demonstrated that the hydrodynamic diameters of nano-TiO2 are significantly greater in phosphate buffered saline (PBS) than in water and that their observed sizes are often significantly larger compared to the quoted particle size. The agglomerate size is generally more than 100nm and is occasionally even more than 1µm.7 It has been observed that both adsorbing multiply-charged ions (e.g. pyrophosphate ions) onto the TiO2 nano particle surface and coating nano crystals with polymers (e.g. polyethylene glycol) suppressed agglomeration and stabilized the dispersions. However the breakage of agglomerates into singles might not happen during real exposure scenarios.53 The behavior of nanoparticles is dependent on their solubility, susceptibility to degradation, and the fact that neither the chemical composition nor the effective particle size of the nano particle will necessarily remain constant over time. This makes it difficult to study and understand the biological Cytotoxicity of any nano particle.
Surface chemistry
Surface chemistry is used to tailor engineered nano particle properties according to their utility. A prerequisite for many applications is the addition of the proper surface coating and functionalization of the nanoparticles to control their environmental interactions. Coatings for nano-TiO2 particles are generally designed to reduce agglomeration/aggregation, target specific cells, modify photo catalytic properties, or improve cosmetic formulations. The coating of nano-TiO2 with polymers (e.g., polyethylene glycol) suppresses agglomeration and stabilizes the dispersion. However, a number of recent studies demonstrated that whatever the size of the nanostructures, they do not freely enter all biological systems. Their behaviour is governed by the functional molecules added to their surface.62 In an aqueous environment, low-surface-energy coatings (hydrophobic) are particularly prone to nonspecific adsorption, such as when proteins denature to expose their hydrophobic core. Hydrophilic coatings (i.e. high surface energy), especially those resulting in a weakly negative or neutral surface charge, are ideal for resisting protein adsorption and cell uptake.63,64 The positively charged surfaces have been shown to engage in strong ionic interactions with the negatively charged cell membrane, which facilitates particle uptake into the phagosomes. Toxicity of TiO2 may be influenced by surface chemistry, but this property is likely to be dependent on the combination of surface modification and cell type.
Adsorption of ions
Most nano particle atoms are on the surface of the particle itself. The surface atoms are unsaturated, can easily bind or interact with other atoms, and possess a high chemical activity. The pH value plays an important role in the adsorption of different ions on oxide surfaces (Figure 3). Solution pH influences the surface active site distribution on metal oxides such as TiO2, and the surface hydroxyl group provides the ability to bind metal ions.65 It is well known that the surface of TiO2 is readily hydroxylated in aqueous solution. When H2O dissociates on a pure TiO2 surface, two distinctive hydroxyl groups are formed.66 The amphoteric surface will be formed because of the acid-base equilibria as shown in equation (1) and (2). Therefore, there are three kinds of surface species, TiOH2 +, TiOH, and TiO-, and their proportion depends on the solution pH and the pzc (pH at point of zero charge) of TiO2.67–69
At a low pH (below the isoelectric point), both As(V) and Cr(VI) take the anionic form,70–72 however the TiO2 surface is positively charged, which increases the extent of anionic adsorption on TiO2 . The nanoparticles at a pH above the isoelectric point adsorb cations (Figure 3) (e.g. Mn(II), As(III), As(V), Fe(III), Cu(II), Cd(II), Ni(II), Zn(II), Pb(II), Cr(VI), Hg(II)).73–79 However, adsorption of ions on the TiO2 surface is not limited to the mentioned examples. These examples were noted because of their possible presence in the effluent discharged from industrial sources. Even at low-level concentrations, the co-existence of nanoparticles with metal ions raises concerns for their enhanced bio toxicity. TiO2-facilitated transportation of Cu, Cd, and As has been reported in carp and Daphnia.80–83 Facilitated transport of adsorbed metals probably occurs when TiO2 nanoparticles enter from the water onto the gill surface and during the consumption of contaminated food sources. Tissues and cellular organelles with a low pH, such as the stomach and the lysosome, may promote the release of such ions. Unfortunately, there is little data explaining the mechanism and extent that the nanoparticles may enable the transport of heavy metals into the environment.
This capability to adsorb trace amounts of chemicals (e.g. Fe) may initiate reactions (e.g. Fenton reactions) and alter the electronic state of the particle’s subsequent catalytic reactions.84 Titanium dioxide is a widely used inorganic component in formulations of stay-on cosmetics, including lipstick, sunscreen and face powder. The potential hazard of trace metal impurities in this matrix should be addressed, since the possible presence of metals raises the issue of nano-TiO2 acting as a magnifier for heavy metal (cations or complexes) pollution.
Solubility and surface impurities
Enhanced photo catalysis cannot always be associated with toxicity. For example, it has been shown that the release of free cadmium from Cd Se nanoparticles is responsible for cytotoxicity in vitro85 rather than the photo activity or nanoscale. Similarly, Brunner et al.86 observed a relationship between material solubility and the cytotoxic response to a range of oxide nanoparticles, with more soluble compounds like ZnO showing greater acute toxicity than those with much lower solubility such as TiO2. The main toxicity of these nanoparticles was shown to be due to surface impurities,87 which often occur as a result of the synthesis process. The use of titanium alkoxides as synthesis precursors,88–91 as well as the use of acetic acid,89 alcohol,92 oils,93 and other organic solvents88,91,92 as reaction media are examples of such sources. Post-synthetic stabilization of nanoparticles through the use of surfactants also introduces extraneous material which may interfere with the accurate observation of the toxicity phenomenon.
Radical formation
Because TiO2 is an efficient photo catalyst in the presence of water, ROS (reactive oxygen species) or “free radicals” are produced as a consequence of UV light exposure. Quantitative studies for OH radical formation have been performed.94 The formation of OH radicals from TiO2 varied according to crystal size and form. Irradiation of anatase produced large numbers of OH radicals in TiO2 in a dose-dependent response to UV, but rutile (90nm) showed less OH radical generation. OH radical generation was significantly influenced by crystal size, but the optimum size was different between both TiO2 forms. Non-UV-induced free radical formation at the surface of nano-TiO2 has been reported by Fenoglio et al.95 Although free radicals were not detected in solution, anatase and rutile-generated carbon center free radicals were found by the cleavage of sodium formate in the presence of H2O2. The presence of trace iron at the surface of TiO2 was cited as a possible cause for the generation of certain kinds of free radicals via a Fenton reaction.
Photo catalysis
In a photo catalytic system, a photo-induced molecular transformation or reaction takes place at the surface of the catalyst. The photo catalytic reaction is initiated when a photo excited electron is promoted from the filled valence band (VB) of the semiconductor photo catalyst to the empty conduction band (CB), where the energy between the two bands is called the “band gap.” The absorbed photon energy (hv), must or exceed the band gap of the semiconductor, to promote the electron and leave behind a positively charged hole in the valence band. Thus both the electron and whole pair (e−+ h+) are generated (equation 3).
The photo generated electrons and holes that do not immediately recombine can migrate to the particle surface and participate in reduction and oxidation processes. Such reactions might include oxidation of adsorbed compounds, oxidative damage to cells, modification of enzymes and sensitive thiols, or transformation of metal ions to a higher or lower oxidation state (Figure 4). To achieve a higher photo activity, it is necessary to minimize the rate of the recombination process which will increase the lifetime of separated electron-hole pairs, such that more electron transfer can occur from the surface to the adsorbed species.96,97
Photo activity is affected by a number of factors such as particle size, crystal structure, incident light intensity, solution pH, and the particle preparation method. Crystal structure and particle size are considered to be the more important factors that determine photo activity. Many researchers have reported that anatase nanoparticles have a higher photo activity than rutile.27,98–100 Others have reported that anatase with a small amount of rutile has a higher photo activity than that of the pure anatase.101 It is interesting to note that size-dependent photo catalytic activity does not increase monotonically with decreasing size but rather passes through a maximum significantly below 100 nm. Optimum sizes are thought to result from competing effects of the particle size on light absorption and scattering efficiency. However, as the particle size is lowered below a certain limit, surface recombination processes become dominant because most of the electrons and holes are generated close to the surface, and surface recombination is faster than the interfacial charge carrier transfer processes. This finding is probably why there exists an optimum particle size for maximum photo catalytic efficiency.38,96,101
Photo catalytic transformation of organic compounds
Photo catalytic decomposition of toxic and non-biodegradable organic compounds using TiO2 as a photo catalyst is an important component in the field of advanced oxidation technologies.96,102–104 Many publications report that test compounds are mineralized into harmless byproducts, but the measurement of compound disappearance is not sufficient to ensure the absence of by-products. The generation of a variety of organic intermediates (in some cases more toxic and persistent than the starting substrate) during heterogeneous photo catalysis has been noted if the treatment is not continued to complete mineralization. For example, increased toxicity of the byproduct has been observed for melamine, diclofenac, azo dyes, and other compounds during TiO2 photo catalysis.105–108
Photo catalytic transformation of metal ions
Photo catalysis can convert the ionic species into metals and deposit them over a semiconductor surface, or transform them into soluble species under thermodynamically favorable conditions.109–111 Metal ions can be reduced or oxidized by the photo catalytic activities of TiO2 (Figure 4).
If the solution contains a metal ion (e.g. Hg(II), Cu(II), Cd (II)) of appropriate redox potential, the conduction band electrons can reduce the species to a lower oxidation state (Equation 4). Alternatively, metal ions (e.g. Pb(II), Mn(II), TI(I) , Cr(VI)) can be oxidized by holes or hydroxyl radicals to a higher oxidation state (Equation 5).
The adsorption, desorption and photo catalytic transformation of toxic heavy metals by TiO2 may dramatically affect the facilitated transport ability of nanoparticles in different compartments of the environment. This ability of TiO2 has been exploited for the removal of heavy metals from wastewater, for example.
Photocatalysis versus photostability
Researchers have been interested in the modification of the electronic and optical properties of nano-TiO2 for its efficient use in water and air treatment and cosmetic formulations. Some researchers have attempted to enhance photo catalysis to decompose organic substances, solar cell production and hydrogen synthesis under visible light. Others have attempted to develop techniques to minimize the photo catalysis of nano-TiO2 without altering its UV screening ability for its use in cosmetics.
Approaches to improve photo catalysis
Surface spots on TiO2 nanoparticles can act as electrodes with both oxidation and reduction processes occurring on them. The surface states and the electron-hole pair recombination of TiO2 determine the overall quantum efficiency for interfacial charge transfer. The balance between the recombination and the trapping of charge carriers, followed by the competition between the recombination of the trapped carriers and the interfacial charge transfer96 are major factors in the efficiency. Therefore, improved charge separation and inhibition of charge carrier recombination is key to improving the overall quantum efficiency for interfacial charge transfer.112 Modification of particle properties by selective surface treatment96 is a common route to this goal.
The foremost limitation of TiO2 as a photo catalyst is the fact that it requires UV irradiation to function. The band gap (i.e. the amount of energy required to free the outer shell electron to become a mobile charge carrier) of anatase TiO2 is 3.2eV (equivalent to 387nm and lower wavelengths), which makes the utilization of solar and indoor light inefficient for photo catalysis because only a small percentage of the available radiation can be used.
Visible light activity has been induced in TiO2 by surface modification using sensitization by dye, polymer, semiconductor particle coupling (Bi2S3, CdS, CdSe, V2O5), and band gap modification by doping (intentionally introducing impurities) with a transition metal (Fe, V, Cr, Mn, Co, Ni and Cu) and nonmetals (halogen, N, , S, B and C).26 Band gap tailoring by doping has been one of the most efficient and frequently used approaches. These modifications of TiO2 decrease the band gap energy or create new energy levels in the band gap (Figure 5A). An appropriate dopant metal (e.g. Mn and Fe ions) causes the absorption edge of the TiO2-based nano-composite to “red-shift” (i.e. to longer wavelengths) (Figure 5B), which makes the materials catalytically active in the visible light spectrum. When metal ion dopants are used, the modification does not depend solely on the type of dopant but also on its concentration and distribution within the particle. The visible light-sensitized nano-TiO2 particles are widely used in research on environmental cleanup technologies. It is quite reasonable to assume that loading nano-TiO2 with different chemical species may produce a completely different eco toxicological impact compared to unmodified particles.
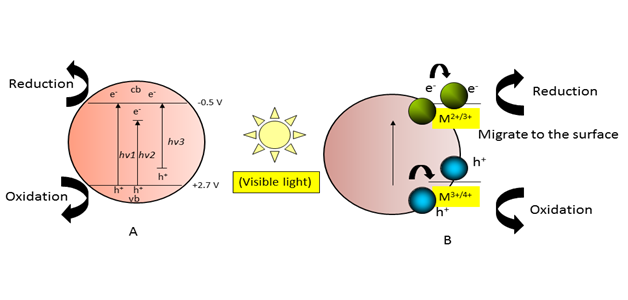
Figure 5A Photo catalysis under visible light Doping-induced intermediate band states in TiO2; hv1: pure TiO2; hv2: metal-doped TiO2, and hv3: non-metal-doped TiO2.
Figure 5B Metal ion-doped nano-TiO2 photo catalyst.
In addition to UV light, visible light was shown to increase the toxicity of nano-TiO2 (carbon-doped TiO2 and TiO2 modified with platinum [IV] chloride complexes) towards bacteria and fungi.113 Independent of light, copper-doped nano-TiO2 particles are able to significantly reduce the growth of certain bacteria compared to copper ions alone. This may be the result of the particles penetrating the cell membrane and releasing copper ions inside the cell.114–117
Approaches to reducing photo activity of nano-TiO2
Because photo catalytic activity is one of the mechanisms for toxicity, it has been proposed to eliminate it by blocking the availability of reactive surface species. Much effort has been made to prepare titanium dioxide samples that are photo chemically inactive but still capable of protecting against UV radiation. Some sunscreens have used titanium dioxide particles that have been modified with inert coatings or doped with certain ions, presumably in an effort to prevent free radical damage to skin. However, current FDA regulations of TiO2 in topical sunscreens do not specify the crystalline form and do not require proof of photo stability (or lack of photo reactivity).118
Surface coatings
TiO2 adsorbs and scatters UV radiation,119 making it a desirable active ingredient in sunscreens where transparency, good dispersibility, and low photochemical and catalytic activities are the desired features. These qualities are acquired by modifying cosmetic-grade nano-TiO2 particles in various ways without disturbing the sunscreen’s ultraviolet-light ray-shielding properties. These modifications include organic (alkoxytitanates, silanes, and methyl polysiloxanes) and inorganic (alumina, silica, and zirconia) surface coatings.120–125 These modifications aim to minimize or eliminate the potential reactivity of photo activated TiO2 particles by quenching and/or reducing the reactive species generated before they can interact with the other ingredients in a formulation or with the skin itself. The labels on sunscreens generally do not indicate which crystal forms of titanium dioxide are present or what types of coating are used. The material used for the surface coatings, its thickness, its chemical purity, or the use of multiple coatings further increases the heterogeneity of the utilized TiO2 nanoparticles,126,127 The matrix in which the nano-sized TiO2 particle resides contributes another factor. The nanoparticles can, for example, be fixed in a matrix, such as the silica beads with nano-sized TiO2 particles from the Sunjin Chemical Corporation, or be bonded together via a surface coating.126
The presence of coatings also adds to the uncertainty about the effectiveness of efforts to reduce unwanted photo activity, and the durability of the coating layer during its life-cycle.128 Some researchers have characterized silica, zircon and alumina as charge transfer catalysts,129 which are solids that have the ability to trap reactive electrons (e−) and electropositive holes (h+),130 and their porous structures facilitate the access of reactant molecules to active surface sites.131 The researchers prepared nano composites of SiO2, ZrO2 and Al2O3 in TiO2,132–135 which displayed improved photo catalytic activity versus pure TiO2.135–139 The factors described above lead to a large diversity in nano-sized TiO2 particles used in cosmetic sunscreens. We must recognize that while many coatings are labeled as being environmentally labile or degradable,16 the partial or complete collapse of the coated material may modify the electronic properties of TiO2 and an initially nontoxic material may become hazardous after shedding its coat.
Metal-doped TiO2
The properties of the nano-TiO2 can be changed as a consequence of production methods or by intentionally contaminating (doping) the crystal structure with other metals.140 For example, manganese doping is used by Oxonica- Croda to reduce the unwanted photo activity of the nano-sized TiO2 marketed under the name Optisol.141 Optisol is composed of ultrafine titanium dioxide-doped manganese (0.7%).142 It is not clearly defined whether this is given in atomic or weight percentage. On the other hand, many researchers reported that manganese-doped nano-TiO2 is able to absorb visible light and operate as a photo catalyst, even under visible light irradiation.143,144
The presence of metals may have the ability to modify the electronic state of TiO2. A metal particle on the surface may act as an electron trap, increasing the separation between the electron and whole and delaying recombination. A metal dopant may also increase whole mobility, which also diminishes the recombination rate.145 Iron oxide (Fe2O3) pigments are added occasionally to give the cosmetic a brown tint to improve the appearance of the sun-care product.146,147 However, one should better understand the events occurring at the nanoscale when adding metals and their oxides to cosmetics that also contain semiconductor nanoparticles. Overall, studies show that modified TiO2 particles that are specifically developed and marketed for sun care, skin care, and color cosmetic formulations still retain photo catalytic activity.148–150 Coatings with alumina and doping with manganese have been suggested to be safe alternatives to other forms of modifications.148,151 Whether photo catalytic activity is still present during user application (i.e., inside a cosmetic formulation in the presence of other ingredients and applied to skin) is open to question. This results in more uncertainty, which forms a barrier to the assessment and management of the risks of nano materials.
Interactions among bio-physicochemical variables
Nanoparticles may undergo surface modification immediately after synthesis.152 The studies using only pristine nano-TiO2 particles may not be relevant for assessing the mechanism of toxicity and predicting behavior. In fact, proper attention needs to be given to the physicochemical properties, modifications and events occurring at the boundaries as a result of interactions (Figure 6).These events are determined by the physicochemical properties like size, shape, impurities, agglomeration, pH, surface chemistry, coating, photo activity, and formation of reactive species (both ex-vivo and in vivo) by nano-TiO2. The release of adsorbed substances and change in the agglomeration state of the nano-TiO2 inside the cell could initiate cytotoxicity and immune responses of varying degrees. Reactive oxygen species products, whether inside or outside the cell, can be key factors in nano-TiO2 toxicological effects. Before the internalization of the nano-TiO2 by a cell, photo catalytic activities may transform the initially harmless compounds into carbon-centered radicals (R*, RO*, ROO*). Photo activation of certain biomolecules may contribute to nano-TiO2-mediated toxicity.153 Most reactions yielding adverse responses or inhibiting toxicity take place at the nano-biointerface where the particle surface, including all adsorbed matter, is in contact with the cell and tissue.
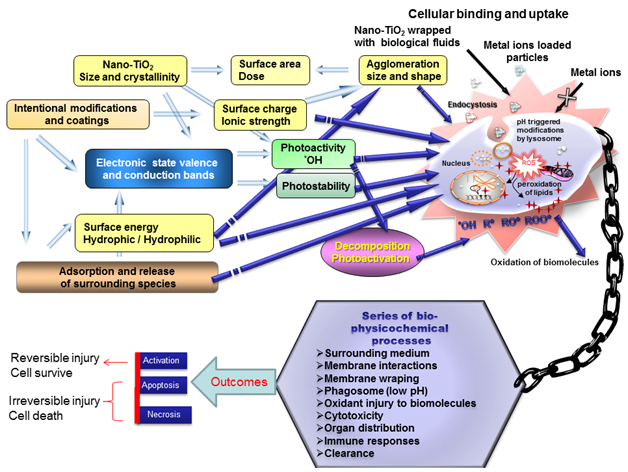
Figure 6 Schematic representation of complex dynamics of nano particle physicochemical properties may cause eco/cytotoxicity.
Proteins are viewed as the primary and most important player in mediating solid organism interactions. The proteins and salts which are included in the biological medium will be adsorbed onto nanoparticles,87 within a brief period of time.154 The adsorption of proteins onto nanoparticles is important for understanding their cytotoxicity. A full characterization of protein adsorption on a nano structured surface should consist of a controlled variation of the following parameters: nanoscale morphology,155 pH and ionic composition of the surrounding medium, protein type and concentration. Curved nano particle surfaces provide extra flexibility and enhanced surface area for the adsorbed protein molecules compared to planar surfaces.156–158 When dispersed in culture medium, some metal oxide nanoparticles can absorb proteins such as serum albumin, in a manner referred to as a “protein corona”.159–161 Moreover, the pre-treatment of TiO2 particles at pH 7 with divalent ions such as Ca2+ and Mg2+ increased the adsorption significantly.162 The possible explanation could be the presence a negative charge on serum albumin at a neutral pH163 and the presence of a calcium binding site, with the albumin bound to TiO2 through Ca2+. The adsorption could be particle size and time dependent.164,165
Transport of the nano materials to the cells precedes the materials’ uptake, and can be modeled by convection, sedimentation and diffusion mechanisms. Transport is strongly influenced by the intrinsic physical factors like size, shape, surface charge, agglomeration and factors related to exposure like number concentration and time. Most of the reactions yielding adverse responses or inhibiting toxicity start at the nano-bio interface (i.e. where the particle surface, including all adsorbed matter, contacts the cells and tissues).7 The uptake mechanism is not well understood at present. Nanoparticles have been found within cells, either free-floating in the cytoplasm or enclosed by a membrane.172–174 The precise mechanism by which TiO2 crosses the selectively permeable barrier of the plasma membrane is a question that must be considered on an individual basis because different cells utilize different nanoparticles uptake pathways depending on variations in size, charge, and surface reactivity. Also, different uptake pathways are associated with different intracellular fates of the internalized nano materials. Phagocytosis is the predominant method of internalization employed by immune cells, such as macrophages and neutrophils. Endocytosis is present in almost every cell type and can proceed through four distinct pathways: (i) clathrin-mediated endocytosis, (ii) caveolin-mediated endocytosis, (iii) macropinocytosis, and (iv) theclathrin/caveolin-independent pathway.175 Not every cell possesses all mechanisms. In addition, passive uptakeis a possible mechanism of cellular entry for some small molecules.
The predominant endocytic pathway in cells is clathrin-mediated endocytosis, which proceeds via the formation of clathrin-coated membrane invaginations that are eventually closed off to form clathrin-coated vesicles and endosomes. Endosomes formed in the clathrin pathway undergo acidification and are eventually destined for lysosomal degradation. The pathways of caveolin-mediated endocytosis and macropinocytosis have slower kinetics than the clathrin-mediated pathway, but the endosomes formed by these two pathways are not directed to the lysosomes. The fourth pathway is not well characterized and is known simply as the clathrin/caveolin-independent pathway.176,177 Phagocytosis is apparently not a major contributor to nano particle uptake because the inhibition of phagocytosis using cytochalasin D (cytD) in macrophages abolished the uptake of micrometer-sized particles, but not of 0.2μm and 0.1μm sized particles. Geiser et al.178 noted that the intracellular nanoparticles were not membrane-enclosed and concluded that non-phagocytic and non-endocytic mechanisms might also be responsible for their uptake. Other reports have proposed that nanoparticles may be sequestered in endosomes, which could be the result of all three major pathways of endocytosis.179,180 In these studies, as in other nanoparticle uptake studies, there was a clear correlation between the nanoparticle localization in the cell, the “availability” of uptake pathways, and the cell type.
Early endosomes formed by the clathrin, caveolin, and macropinocytic processes pursue a defined pathway that leads to the formation of late endosomes. This formation is followed by sorting within multi vesicular bodies and, finally, fusion with degradative lysosomes.181,182 Notably, TiO2 nanoparticles have been localized in endosomes as well as to multi vesicular bodies.179 Several in vitro examinations concerning cellular responses induced by ultrafine TiO2 particles have been reported and mainly involve oxidative stress. These studies suggest that ROS are involved in the cytotoxic effects of TiO2 with two mechanisms, namely extra cellular ROS generation and intracellular ROS induction. Compared to photo excited TiO2particles, the cytotoxic effect of non-photo excited TiO2 particles was lower.47,183 However, a change in certain physical and chemical properties led to a change in biological activity.3
Influence of interactions with other materials
Ex vivo testing determined that sunscreen nanoparticles remain on the surface of the skin in the stratum corneum among keratinized cells and do not reach the underlying viable cells. Moreover, the issue of the ROS generated by nanoparticles in sunscreens interacting with other materials has also been raised, including the unattractive hand- and finger-shaped blemishes seen on coatings of pre-painted steel roofing sheets after they have been handled by workers wearing nano particle-containing sunscreens.149 Despite it is widely investigated photo catalytic properties, which indicate that large numbers of redox reactions can occur on the surface during UV exposure, titanium dioxide has always been considered to be a harmless and innocuous ingredient when added as a filter to sunscreen preparations or added as a whitener to many food and cosmetic products.184–186 A number of recent studies have revealed that under UVA irradiation, TiO2 may catalyze the degradation of biomolecules (Table 1). Additionally, they have the potential to destroy peroxidase enzymatic activity187 and cause in vitro photo toxicity.188,189 Several studies indicate that titanium dioxide extracted from commercial sunscreens is equally effective in causing DNA damage (Table 1).
TiO2 particle |
Experiment |
Outcomes |
0.45μ Manatase |
Irradiation of solutions containing calf thymus DNA |
Significant levels of photo oxidation of nucleic |
Extracted From Commercially Available Sunscreens |
Oxidation of organic material (phenol), |
TiO2 stimulates oxidation of organic materials |
P25 |
The fate of nitrogen in various amino acids |
Nitrogen in the amino acids are photo converted |
Anatase or Rutile |
Bovine serum albumin (BSA) protein nitration |
Protein nitration was observed and identified |
Table 1 Nano-TiO2 stimulated degradation of biomolecules
TiO2 can interact with organic compounds that are present in their vicinity; for example, reactive carbon-centered radicals are generated during the mineralization of organic sunscreen components.190,191 In light of these results, it is somewhat surprising that the possible interaction between sunscreen organic components and titanium dioxide has not been studied with the same enthusiasm as was done for the skin penetration studies. In order to properly investigate the photo toxicity of sun protection products, not only should the filters be considered but also the other components that despite being photo chemically inactive, may participate in the generation of harmful species, such as preservatives (isothiazoline family, parabens mixtures, and formaldehyde donors), anionic surfactants, oils, water or alcohol based gels, or antioxidants, which are all widely used in sunscreen preparation.
Skin penetration
Dermal absorption of chemicals must be considered during risk evaluation.192–194 The skin is the largest organ of the body and accounts for over 10% of the body’s mass, and plays an important role as a barrier against the external environment. The skin functions in protection, maintaining homeostasis and metabolism. Four mechanisms of penetration across the skin have been identified and are dependent on the physicochemical properties of the compound. These mechanisms include an intercellular, a transcellular, and two trans appendageal (hair follicles and sweat glands) mechanisms. Several recently published reviews provide excellent overviews of the diffusion of micro- and nano sized ZnO and TiO2 through the skin.195–198 A large number of studies suggest that these particles do not penetrate human skin beyond the superficial layers of the stratum corneum.3,195–200 Skin exposure to nano particle-containing sunscreens leads to incorporation of TiO2 and ZnO in the stratum corneum, which may alter certain properties due to particle-particle, particle-skin, and skin-particle-light physicochemical interactions.195 Overall, the weight of scientific evidence suggests that insoluble nanoparticles used in sunscreens pose no or negligible risk to human health196,197,201–210 however there are some discrepancies in the results probably related to differences in techniques and methods, laboratory conditions, and the absence of standardized evaluation protocols.
The reason for these results is unclear based on the observation that most other nano particle types (polymers, metals and carbon nano tubes) permeate the skin. The answer may be that it is possible that the particle agglomeration,211,212 when combined with the particles’ intrinsic hydrophobicity, allows particles to become trapped in the lipid lamella and remain until desquamation or sebaceous secretion removes them from follicles. Efficient transdermal drug delivery has been correlated with skin hydration.213,214 This finding indirectly suggests the importance of accessing the polar pathway in penetrating the skin barrier, which hinders hydrophobic particle penetration. There may be physicochemical factors other than size, surface charge, and surface energy that are important when evaluating nano material penetration (e.g. the nano particle mechanical properties). For example, recent findings in studies that measured the distribution profile of elastic and rigid vesicles (115nm diameter) in human skin identified that elastic particles penetrate deeper under identical conditions.215 Perhaps the particles must be able to fit through the stratum corneum, and metal oxide nanoparticles may be less compliant than metals of semiconductor QDs. Research findings demonstrate that the penetration of TiO2 is negligible in healthy skin. This is important, as nanoparticles appear to be unable to reach the living cells present within the deeper skin layers, and therefore their propensity for toxicity is anticipated to be minimal. Future studies should consider the fate of particles in skin models, taking into consideration that sunscreens are often applied to burnt, damaged, or diseased skin where the barrier function of the stratum corneum may be impaired.49,208 More research is needed to resolve this issue and to gain a quantitative understanding of the extent and mechanisms of nano particle skin penetration.
Most of the adverse effects, including inflammatory response, Genotoxicity, lipid peroxidation, oxidative stress and changes in enzyme activity have been attributed to the small size of TiO2 particles. Particle size does play a contributing role in toxicity, because reduced size corresponds to increased particle numbers and enhanced surface area for a given particle mass. However, the reactivity of the particle surface and interactions with the surrounding matrix can also lead to complex and unexpected phenomena arising from the interplay of many factors. Particle-cellular interactions and particle-environmental interactions can have a significant influence on reactivity modifications generating toxic species, with their subsequent impact on health and the environment. The large specific surface area of the nanoparticles is very effective for capturing low concentration chemical species such as metals. The presence of impurities not only influences the photo catalytic properties of TiO2, but may be helpful in the transportation and accumulation of trace elements in both cellular- and environmental-related studiesin which pH could play a very important role by the change of agglomerate size, and by adsorption and release of trace metals. The interaction between nano-TiO2 and a biological system and its surroundings are based on their inherent physicochemical properties and the modifications made to the nano-TiO2. A largely ignored aspect in the literature is the photo catalytic ability of nano-TiO2 to mineralize organic components and, in the case of partial decomposition, to form reactive intermediates. These reactive species have the potential to cause oxidative injury ex vivo or in environmental-related scenarios. It becomes a considerable challenge for nanotoxicologists to determine whether this toxicity is due simply to size or to the reactivity of nanoparticles with the surrounding medium. Alternatively, the nanoparticles may only be playing a role in the transportation and accumulation of certain chemical species, or the toxicity may be the combination of different processes. The challenge for toxicologists is to identify key factors or tests by which nano particle toxicity can be measured or predicted, and these data must be reproducible in other laboratories. Multidisciplinary efforts will be required to fully understand the underlying mechanism of nano material toxicity.
Careful examination of the classical (particle size, surface area and dose) and non-classical interactions (size-related properties and altered reactivity) of nano-TiO2 may be helpful in developing safer consumer goods, such as sunscreen. Nano-based sunscreens contain particles in the range of 10 to 100nm200 and contain both anatase and rutile crystals, either alone or in combination.216 Most studies have revealed the absence of cutaneous penetration. Smaller, doped, coated, and more dispersed TiO2 particles were demonstrated to have superior UV attenuation, due to enhanced UV scattering. The problem of undesirable photo catalysis is addressed by applying surface treatments to the crystals, selecting a less photo reactive form (rutile), or adding antioxidant ingredients to the formula. Recent research has identified that the surface coatings on nano-TiO2 in many sunscreens may be unstable or ineffective.149 However, doping rutile nano-TiO2 with manganese was reported to increase UV absorption, reduce free radical generation, and increase its free radical-scavenging behavior.142,151 Perhaps an important question is whether regulatory authorities should be discriminating between anatase and rutile titanium dioxide particles, or between doped and un-doped particles, in sunscreens. There is an urgent need for an international consensus for determining the status and safety requirements of these products and their ingredients.
None.
The author declares no conflict of interest.

©2015 Tanvir, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.