MOJ
eISSN: 2379-6294


Research Article Volume 4 Issue 4
Expertox Laboratory, France
Correspondence: Stephane Pirnay, Expertox Agency and Laboratory, 14 rue Godefroy Cavaignac 75011 Paris, France, Tel 33(0)9 8107 8503
Received: April 19, 2018 | Published: August 31, 2018
Citation: Vaugelade SD, Taillandier C, Thomas C, et al. Validation of analytical method for the quantitative determination of preservative acids allowed in Eco label cosmetic products. MOJ Toxicol. 2018;4(4):304–307. DOI: 10.15406/mojt.2018.04.00117
Preservative is a natural or synthetic substance incorporated into cosmetics in order to avoid chemistry (oxidation) or microbiologically alterations. It prevents microorganisms’ proliferation such as fungi that may create skin allergies in contact with the skin. These preservatives are essential for any cosmetic product, organic or not.
The origin of preservatives may be synthetic or natural. To be used in a cosmetic product in Europe, they must be listed in Annex V of EC Regulation N°1223/2009. There are fifty original synthetic antimicrobial preservatives authorized in Europe.1
Preservation of a natural cosmetic product should logical be ensured by a natural way. Because of the difficulty of defined properly the efficiency of natural preservatives (absence of minimum inhibitory concentration (MIC), absence of toxicological data) some synthetic preservatives are authorized by labels for their occurrence in nature, their low toxicity and their limited irritant or allergenic reactions. The main synthetic preservatives allowed in natural products are: benzyl alcohol, benzoic acid and its salts, dehydroacetic acid and its salts, salicylic acid and its salts, sorbic acid and its salts (Table 1).
Country |
Label |
Benzoic acid |
Sorbic acid |
Salicylic acid |
Dehydroacetic acid |
Benzyl alcohol |
Propionic acid |
Formic acid |
Germany |
BDIH |
X |
X |
X |
X |
X |
||
France |
Ecocert/Cosmebio |
X |
X |
X |
X |
X |
||
France |
Nature & Progrès |
X |
X |
X |
||||
UK |
Soil association |
X |
X |
X |
X |
|||
France |
Natrue |
X |
X |
X |
X |
X |
X |
|
France |
Cosmos |
X |
X |
X |
X |
X |
Table 1 Synthetic preservatives authorized by European Eco labels.2‒5
These compounds are allowed in finished cosmetic products by the European Commission, in Annex V of the cosmetics Regulation No. 1223/2009 with maximum permitted concentrations between 0.5% and 2.5% (Table 2). In this context, EXPERTOX laboratory has developed and validated an LC-UV analytical method for the determination and quantification of four acid preservatives in cosmetic products as part of a quality control and in compliance with the Regulation No. 1223/2009. Benzyl alcohol quantification assay by GC-MS was already validated by the laboratory to determinate allergens in cosmetic product.6 The compliance within the authorized concentrations in the final product is intended to ensure good preservation and stability of cosmetics, and there ensure consumer safety.
Substance |
Product, body part |
Maximum authorized concentration |
Limitations and requirements |
Conditions of use and warnings which must be printed on the label |
Benzoic acid and its sodium salt |
Rinse-off products, except oral care products |
2,5% (acid) |
||
Oral care products |
1,7% (acid) |
|||
Leave-on products |
0,5% (acid) |
|||
Salicylic acid and its salts |
0,5% (acid) |
Not to be used in preparations for children under three years old, except for shampoos |
Not to be used for children under three years old (1) |
|
Sorbic acid and its salts |
0,6% (acid) |
|||
Dehydroacetic acid and its salts |
0,6% (acid) |
Prohibited in aerosol dispensers (sprays) |
||
Benzyl alcohol |
1% |
|||
Table 2 Restriction limits in finished products
Standards, reagents and solvents
All solvents were analytical grade or HPLC grade. The selected compounds used in the present work are: Benzoic acid (BA), Sorbic acid (SA), Salicylic acid (SAA), Dehydroacetic acid (DHA), Benzyl alcohol (BA). They were acquired from Sigma-Aldrich (Saint-Quentin Fallavier, France). Methanol was supplied by Sigma-Aldrich (Saint-Quentin Fallavier, France). Citric acid and Acetate ammonium were supplied by Fisher Chemical (Illkirch, France) and VWR (Fontenay-sous-Bois, France). Purified water from Milli-Q was used (Millipore).
LC/UV conditions
The liquid chromatography was performed by an Agilent 1220 Infinity LC system including a degasser and a variable wavelength UV/Vis detector, connected to Open Lab Station. The LC columns were a ZORBAC Eclipse Plus C18 (4.6x150nm, 3.5µm). The optimal chromatographic separation of the acid preservatives was obtained under the following conditions: the mobile phase in isocratic composed of a buffer mixture methanol-acetate (pH 4.4) (35:65v/v) at a flow rate of 1 mL/min, 5µL of the sample was injected, and the wavelength was set at 230nm.
Calibration standard solutions
Preparation of stock standard solutions: For the preparation of preservatives stock standard solutions, separate stock solutions of 100µg/mL were prepared by dissolving an appropriate amount of each compound in methanol. Mixed stock standard solutions of 10µg/mL (BA, SA, SAA, and DHA) were prepared by mixing 1 mL of each stock solution and fill up to 10mL.
Calibration curve of standard solutions: The calibration curves are obtained by diluting mixed stock standard solutions to six points of concentrations between 100 and 1000µg/mL.
Assay validation
Validation of the chromatographic method is based on ISO 1278:2011 concerning the validation of analytical results using chromatographic techniques in cosmetic. Validation criteria’s are: range linearity, accuracy, precision, repeatability and quantification/detection limits.7,8
Extraction of preservatives from cosmetic samples
About 1g of cosmetic product was weighed into a plastic falcon tube, 10mL of methanol was added, after vortex the tube was immersed for 30minutes in an ultrasonic thermo stated bath at 60°C to melt any lipid phase and to facilitate the extraction of active compounds into the methanol phase. Then, the tube was centrifuged for 20 minutes at 3000rpm. The supernatant was filtered with 4µm filter and 1mL volume of the supernatant was introduced in sample vial. Some matrixes have to be diluted to fit with the validated linear regression curve.
Chromatography
As shown in Figure 1, good chromatographic separation was achieved for all the analytes. SAA, BA, SA, and DHA were separated in 10minutes.
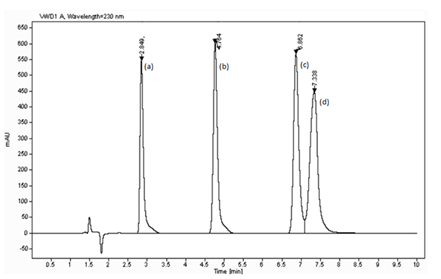
Figure 1 Chromatographic separation (a) Salicylic acid, (b) Benzoic acid, (c) Sorbic acid, (d) Dehydroacetic acid.
Linearity
Good lineraity was occurred within correlation square coefficient greater than 0.99, indicating a linear relationship from 10 to 1000µg/mL for all compounds. Linearity range and squared correlation coefficients are presented in Figure 2.
Precision, accuracy and repeatability
To evaluate the precision and recovery, six samples containing 100, 500 and 1000µg/mL of analytes were analysed. For the repeatability, one sample is analysed six times. Results are summarized in Table 3. Precision, recovery and repeatability for all analytes were 0.13%-4.66%, 0.1%-19.5% and 0.35%-4.33%, respectively. Results are different depending on analytes; nevertheless, the validation data was acceptable for all analytes.
Limit of detection (LOD) and quantification (LOQ)
The sensitivity of chromatographic methods is determined by the LOD and LOQ. They were calculated as the lowest compound concentration. The LOD was 50µg/mL, and LOQ was 100µg/ mL for all analytes (Table 3).
Analytes |
Expected concentration (µg/mL) |
Observed concentration (µg/mL) |
Precision(%) |
Recovery (%) |
Repeatability (%) |
BA |
100 |
94,26 |
1,63 |
5,74 |
2,15 |
500 |
510,47 |
0,26 |
2,10 |
0,88 |
|
1000 |
994,35 |
0,42 |
0,56 |
0,51 |
|
SAA |
100 |
85,91 |
0,13 |
19,52 |
2,97 |
500 |
518,64 |
1,14 |
1,46 |
0,82 |
|
1000 |
990,12 |
0,27 |
0,58 |
0,79 |
|
SA |
100 |
80,48 |
2,86 |
19,52 |
2,34 |
500 |
507,31 |
1,16 |
1,46 |
0,51 |
|
1000 |
994,20 |
0,75 |
0,58 |
0,54 |
|
DHA |
100 |
104,27 |
4,66 |
4,27 |
4,33 |
500 |
504,36 |
2,50 |
0,87 |
1,62 |
|
1000 |
999,01 |
0,27 |
0,10 |
0,35 |
Table 3 Precision, recovery and repeatability results
Extraction
All preservatives were extracted from different types of products (cream, gel, lotion). Comparison between sample spiked before and after extraction have shown recoveries >90% for all analytes.
Application
This validated method applied by our laboratory in order to monitor the preservative concentrations in eco label cosmetic products in compliance with the regulation 1223/2009. Quantitative determination by LC/UV of the main synthetic preservatives in a commercial cream marketed in Europe is presented in Figure 3. Result has shown that dehydroacetic acid was found at a concentration of 647ppm. No other preservatives have been detected with this method. DHA concentration is in compliance with the regulation 1223/2009 (<0.6%).
To ensure the preservation of cosmetics and consumer safety in compliance with the authorized concentrations as set up by the Regulation 1223/2009, EXPERTOX laboratory has developed and validated a quantitative determination and extraction methods of 4 main synthetic preservatives authorized by the organic labels: benzoic acid, sorbic acid, salicylic acid and dehydroacetic acid.
LOQ is below the regulatory limit concentrations of the 4 preservatives acids, between 0.5% and 2.5%, as part of the quality control in compliance with 1223/2009 Regulation. The analytical results had permitted the validation of all criteria’s which ensure the performance, reliability and quality of the final results. Chromatographic and extraction methods were validated.
Even if the labels focus on the origin, on the green process and on the good toxicological profiles of raw materials, certified organic products do not imply a perfect tolerance as the consumer could think. Preservatives can induce allergenic reactions despite their limited toxicological profiles. The preservation of biological or natural cosmetic products requires the development of new approaches to tend to low-risk microbiological products.9,10 The researches on packaging in order to limit the risk of contamination (one-use, airless ...) is a delicate way since it must remain compatible with the ecological requirements (limitation of packaging). The self-protection concept is increasing, it needs the presence of substances which get bactericidal-fungicidal properties without preservatives such as alcohols (ethyl, benzyl), aliphatic glycols (propylene glycol, glycerine), soaps, some fatty acids (monolaurin lipoaminoacids), antioxidants that work synergistically with traditional preservtives (BHT, EDTA...) and finally, the substances from plants or rich of substances with microbiological properties.
None
Author declares that there is none of the conflicts.

©2018 Vaugelade, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.