MOJ
eISSN: 2379-6294


Research Article Volume 4 Issue 3
1Instituto Nacional de Salud Pública, México
2Department of Environmental Health, Emory University, USA
3Instituto de Investigaciones Biomédicas, Universidad Nacional Autónoma de México, México
4Instituto Nacional de Enfermedades Respiratorias, México
5Instituto Nacional de Ciencias Médicas y Nutrición Salvador Zubirán, México
6Instituto Mexicano del Seguro Social, México
Correspondence: Albino Barraza Villarreal, Instituto Nacional de Salud Publica, Av. Universidad # 655, Col. Santa Maria Ahuacatitlan, C. P. 62100 Cuernavaca, Morelos, Mexico, Tel 5277 7101 2981
Received: May 02, 2018 | Published: May 10, 2018
Citation: Escamilla-Nuñez MC, Barraza-Villarreal A, Hernández-Cadena L, et al. Exposure indices for phthalates and bisphenol A and their potential use in epidemiological studies. MOJ Toxicol. 2018;4(3):111–119. DOI: 10.15406/mojt.2018.04.00098
Exposure to endocrine disruptors such as phthalates and bisphenol A (BPA) are currently an important public health problem. Exposure to these chemicals can result in changes in an organism’s metabolic pathways and cause health problems in the exposed population. The aim of the present study was to generate indices of exposure to endocrine disruptors based on concentrations obtained in maternal urine samples collected during pregnancy. 290 urine samples were collected and analyzed of BPA concentrations and nine phthalate (mono (3carboxypropyl) phthalate, monoethyl phthalate; mono (2-ethyl-5-carboxypentyl) phthalate, mono (2-ethyl-5-hydroxyhexyl) phthalate, mono-n-butyl phthalate, mono-isobutyl phthalate, mono (2-ethyl-5-oxohexyl) phthalate, monobenzyl phthalate, and mono (2-ethylhexyl) phthalate) metabolites were performed. Factor analysis was used to construct the exposure indices. Two indices were identified, and each one contained a group of chemicals who share mechanisms of action and sources of exposure. The first group is made up of the metabolites; mono (2-ethyl-5-hydroxyhexyl) phthalate, mono (2-ethyl-5-carboxypentyl phthalate, mono (2-ethyl-5-oxohexyl) phthalate and mono (2-ethylhexyl) phthalate, which are commonly used to soften plastics and are frequently found in food. The second index contained the following compounds; mono-n-butyl phthalate, monobenzyl phthalate, mono-isobutyl phthalate, mono(3-carboxypropyl) phthalate, BPA and monoethyl phthalate, which are mainly found in solvents, additives, adhesives and cosmetic products. Our results showed strong correlations among chemical substances belonging to the same group, and those from the same source and they share toxicological characteristics. The exposure indices generated could be used to evaluate the effect of exposure to multiple endocrine disruptors in epidemiological studies.
Keywords: phthalate, bisphenol A, endocrine disruptor, pregnancy
BPA, bisphenol A; DEHP, di(2-ethylhexyl) phthalate; DnOP, di(n-octyl) phthalate; DEP, diethyl phthalate; HMW, high molecular weight; LOD, limited of detection; LMW, low molecular weight; MWs, molecular weights; MEHP, mono(2-ethylhexyl) phthalate; MECPP, mono(2-ethyl-5-carboxypentyl) phthalate; MEHHP, mono(2-ethyl-5-hydroxyhexyl) phthalate; MEOHP, mono(2-ethyl-5-oxohexyl) phthalate; MCPP, mono(3-carboxypropyl) phthalate; MnBP, mono-n-butyl phthalate; MiBP, mono-isobutyl phthalate; MBzP, monobenzyl phthalate; MEP, monoethyl phthalate; EDCs, the endocrine disrupting chemicals
The Endocrine Disrupting Chemicals (EDCs), are chemicals that are structural analogs of endogenous hormones and can alter metabolic pathways.1–5 The main compounds classified as EDCs are polychlorinated biphenyls, dioxins, polycyclic aromatic hydrocarbons, phthalates, bisphenol A (BPA), pesticides, alkylphenols, and some heavy metals.1 Currently, in several countries, there are no established standards for control of these chemicals including Mexico,5 However, there is currently some regulation of use of phthalates in the USA, especially in child care products (Consumer product safety commission from U.S.A). Various studies have documented that gestational exposure to phthalates alters the regulation of embryo development, which negatively effects on reproductive health and neurodevelompment of individuals and their progeny.1,4–12 Exposure to BPA has been associated with miscarriage,13 development of gestational diabetes, reduced glucose tolerance, and an increase in plasma insulin, triglycerides, leptin concentrations, and obesity.14
Phthalates and BPA have been detected in the environment because of their use in the manufacture of plastics, where they are included as additives to provide flexibility, hardness, transparency, or durability to the plastic.15,16 These chemicals are also present in many common personal care products, cleaning products, construction materials, home furniture, electronics, food packaging, pharmaceutical products, and insecticides. Consequently, humans are frequently exposed to multiple EDCs.17,18 Phthalates are present in the environment as phthalate acid esters, which are lipophilic. On entering the human body, esterase’s and lipases present in the intestine and parenchyma rapidly metabolize phthalates to their esters.19,20 Some of the primary metabolites in turn undergo hydroxylation, oxidation, and conjugation reactions, before being excreted.21
Phthalates have biological half-lives on the order of hours.21,22 The main phthalates and its metabolites that have been identified in human urine15 include di(2-ethylhexyl) phthalate (DEHP), di(n-octyl) phthalate (DnOP), dibutyl phthalate, diisobutyl phthalate, benzyl butyl phthalate, and diethyl phthalate (DEP).23 Each of these are metabolized to primary or secondary esters. Approximately 75% of DEHP is excreted in the urine within the first 48 h of exposure as mono(2-ethylhexyl) phthalate (MEHP) and other chemicals from oxidative metabolism, including mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), and mono(2-ethyl-5-oxohexyl) phthalate (MEOHP).4,22,24, DnOP is metabolized to mono(3-carboxypropyl) phthalate (MCPP). Dibutyl phthalate and diisobutyl phthalate are metabolized to mono-n-butyl phthalate (MnBP) and mono-isobutyl phthalate (MiBP), respectively.23–27 Benzyl butyl phthalate is transformed into monobenzyl phthalate (MBzP), and the main metabolite of DEP is monoethyl phthalate (MEP).19,23,28
Lipophilic BPA, is synthesized by combination of two moles of phenol and one mole of acetone, and is moderately soluble (120–300mg/L at pH 7).29,30 To date, its metabolism has not been completely characterized, However, this compound has been investigated in international studies for the estrogenic effects.30 Exposure to phthalates and BPA can be transplacental, inhalatory, dermal, and/or oral , with the latter being the most common.30,31 Although, there are a lot evidence of the health risks of exposure to these substances,32 the mechanisms and modes of action of these chemicals are not yet clear. Because humans are usually exposed to mixtures of these chemicals rather than each one in isolation33 and through many different pathways,17,18 current toxicology methods focus on evaluating the effect of mixtures of contaminants on human health. Exposure indices are important for performing this type of evaluation.34 Different authors have used different ways to represent exposure to these substances.34–36 used the sum of the phthalate metabolites from the same parent phthalate. Christensen et al.37 used the cumulative risk index generated by the sum of the ratios between the current level of exposure and the acceptable level of phthalate exposure (reference value). Trasande & coworkers38 considered the sum of the metabolites according to molecular weight for both high and low molecular weight groups. The aim of the present study was to develop exposure indices for phthalates and BPA from urine samples of pregnant women. These indices could be applied for the evaluation of the individual and combined effects of these chemicals on the health of a study population. They could also be used as risk markers in later stages of development in neonates.
Women in the present study were from mother and child pairs who participated in a large randomized clinical trial (National Institute of Public Health/Instituto Nacional de Salud Pública (INSP) Mexico: CI-011 in clinicaltrials.gov: NCT00646360). Participants were recruited at the General Hospital of the Instituto Mexicano del Seguro Social (IMSS) in Cuernavaca, Mexico. Generally, the IMSS enrollees were of middle to low socioeconomic status.39 The study protocol was approved by Emory University’s Human Investigations Board (Atlanta, GA, USA), the Ethic Committee of INSP (Cuernavaca, Mexico), and the IMSS General Hospital’s Human Subjects Boards. All procedures were explained to the participants, who signed an informed consent form. For the present report, we selected a sub-sample of 290 binomials from the 1094 women participants of a cohort. Eligible binomial were those who had complete information.
Determination of phthalate metabolites and BPA
The first morning urine samples were collected from the mothers during the third trimester of pregnancy. Containers prewashed and pollution free were provided to the participants and they were instructed on sample collection procedures. After sampling, 5-mL aliquots were stored frozen at –70°C until analysis. Analysis was conducted to determine the presence of nine monoester phthalate metabolites (MEHP, MECPP, MEHHP, MEOHP, MBzP, MnBP, MiBP, MEP, and MCPP) and BPA in the laboratory of the Emory University of Atlanta, USA. Human urine samples were processed using enzymatic deconjugation of the glucuronides followed by solid-phase extraction. For phthalate determination, 1–2mL urine aliquots were processed. The assay precision was improved by incorporating a 13C4-labeled internal standard for each analyte, and a conjugated internal standard to monitor deconjugation efficiency. The analytes of interest were released in their second conjugated phase and isolated by solid-phase extraction. The samples were analyzed by isotope dilution high-performance liquid chromatography coupled with tandem mass spectrometry (HPLC/MS)The concentration of each analyte was calculated from a calibration curve prepared using a standard. The Limits of detection (LODs) for all analytes were 0.1ng/mL, except for MEHP (0.5ng/mL) and MCPP (0.5ng/mL).40
For quality control purposes, targets for the method, overburdened samples with known levels (n=10% of the samples), standards, and blind samples whose concentrations were compared with known levels were analyzed.
BPA analyses were performed using gas chromatography mass spectrometry according to an established method.41 Phthalate and BPA urinary concentrations are reported both in micrograms per liter of urine and in micrograms per gram of urinary creatinine (µg/g creatinine). Creatinine determination was realized using high-resolution liquid chromatography and the levels were used to correct for urine dilution.
Statistical analysis
All 290 samples of maternal urine were used for statistical analysis of the phthalate metabolite and BPA results. The geometric means and the 50th, 75th, and 90th percentiles of the concentrations of the phthalate metabolites and BPA were calculated. Phthalate metabolite concentrations below of the limited of detection (LOD) were assigned a value equal to the LOD divided by the square root of the assigned value,42 taking into account that the missing values did not exceed 5% of the sample size. Likewise, we used multiple imputation43 for obtaining estimate values of MEHP. Later, these were transformed into a natural logarithm (Ln (corresponding metabolite)) because of the asymmetrical distribution of the data. The multiple imputation is an iterative process of estimation that it is repeat several times for each complete data set, we considered to the analyses the method that uses Markov Monte Carlo chain (MCMC) procedures; we assume that all the variables in the imputation model have a joint multivariate normal distribution. The imputation was evaluated from variance (within, between and total), relative increase in variance, the fraction of missing information and relative efficiency, well as the used of graphs to evaluate the residues, atypical values for each data set imputed single, as well for evaluating the convergence of model. To investigate grouping patterns in the data, we used Pearson correlation matrix and dispersion graphs. For constructing the exposure indices and considering that there is intercorrelations between factors, we used confirmatory factor analysis with the promax procedure, which uses oblique rotation,44 we considered maximum likelihood as method to estimate the parameters of the model and the regression method to extracted factors. The model was examined and evaluated its goodness of fit.
The model of factorial analysis is given for equations:
Where arevariables transformed with log-natural of phthalate metabolites and BPA; common factors; the unique or specific factors and the set of linear the coefficients.
factor loadings.
Analyses were performed using Stata 14 statistical software (StataCorp LP, College Station, TX, USA).
Table 1 shows the sociodemographic characteristics of the study population. Mean age of women was 26.1 years (SD=4.7), more than one-half were overweight (55.2%), and only 55.4% completed secondary school. Parity, 42.8% of women had two deliveries, include current pregnancy and 17.6%, 3 or more. In addition, 34.5% of the mothers they had a low socioeconomic level. Among the nine phthalate metabolites studied, the highest average concentrations (Table 2) were for MEP at 106.8µg/g creatinine (95% confidence interval (95%CI): 88.7, 128.6), MnBP at 77.6µg/g creatinine (95%CI: 66.2, 91.0), and MECPP at 46.8µg/g creatinine (95%CI: 39.6, 55.2). By contrast, the levels of BPA (2.1µg/g creatinine; 95%CI: 1.8, 2.5) and MCPP (2.8µg/g creatinine; 95%CI: 2.4, 3.2) were low. From the correlation matrix (Figure 1), we identified strong (r >88%) and significant (p<0.05) correlations among the metabolites Ln(MEHP), Ln(MECPP), Ln(MEHHP), and Ln(MEOHP), and weaker relationships among the metabolites Ln(MnBP), Ln(MiBP), and Ln(MBzP) (r> 68%, p< 0.05). The high correlations among Ln(MECPP), Ln(MEHHP), and Ln(MEOHP) could be attributed to the fact that three of these metabolites are derived from MEHP. Table 3 shows the correlations between phthalate metabolites and BPA with the resulting factors from multivariate analysis, represented by rotated common factors. Factor one was strongly related to the phthalate metabolites Ln(MEHHP), Ln(MECPP), Ln(MEOHP), and Ln(MEHP), which are derived from DEHP (Table 4), and weakly related to the remaining phthalate metabolites and BPA. Factor two was strongly related to the phthalate metabolites, Ln(MnBP), Ln(MBzP), Ln(MiBP) and Ln(MCPP which are produced by metabolic transformation of DnOP, benzyl butyl phthalate, dibutyl phthalate, and diisobutyl phthalate, respectively. It was weakly related to Ln(BPA) and Ln(MEP), which is derived from DEP, and the rest of the phthalate metabolites.. Therefore, Factor 1 is for phthalate metabolites that are derived in the later stages of metabolism of DEHP and Factor 2 is for a mixture of phthalate metabolites and BPA (Table 3) (Figure 2). Each factors obtained represents an exposure index, which we labeled as 2-ethyl and phthalate mixtures in reference to the rest of the phthalate metabolites or BPA that they contained. The correlation of the promax (3) rotated common factors was 62.6%. Finally in Table 4 shows the physical and chemical characteristics of the metabolites studied.
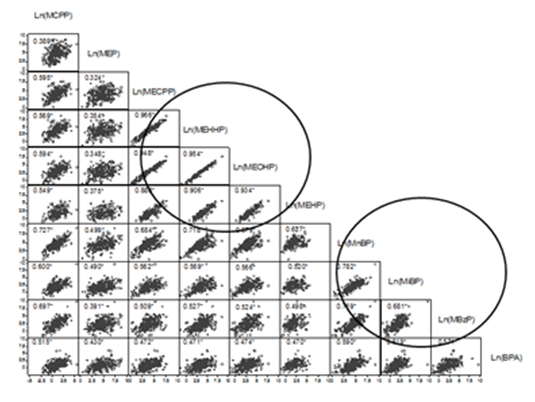
Figure 1 Correlations between phthalate metabolites and bisphenol A in urine from pregnant Mexican women (n =290) on a logarithmic scale. Values correspond to Pearson correlations. *p < 0.05
Variable |
Mean |
(SD) |
Age, years |
26.1 |
(4.7) |
High school (12 y or more), n(%) |
160 |
(55.4) |
Socioeconomic level, n(%) |
|
|
Tertile 1 |
100 |
(34.5) |
Tertile 2 |
104 |
(35.9) |
Tertile 3 |
86 |
(29.6) |
Anthropometric measurements |
|
|
Height, m |
155.0 |
(5.6) |
Weight, k |
62.6 |
(11.4) |
Body Mass Index (BMI), k/m2 |
26.0 |
(4.4) |
Overweight (BMI>24.9), n(%) |
160 |
(55.2) |
Parity, include current pregnancy, n(%) |
|
|
1 |
96 |
(33.1) |
2 |
124 |
(42.8) |
3 |
51 |
(17.6) |
4 or more |
19 |
(6.6) |
Table 1 Characteristics of the study participants (n = 290)
SD, standard deviation
Phtalate parent compound |
|
||||
Geometric mean (95% CI) |
Percentile |
||||
50 |
75 |
90 |
95 |
||
DnOP |
|
|
|
|
|
MCPP |
2.8 (2.4, 3.2) |
2.7 (2.4, 2.9) |
5.7 (4.9, 6.8) |
16.9 (11.6, 20.4) |
25.4 (19.3, 36.8) |
DEP |
|
|
|
|
|
MEP |
106.8 (88.7, 128.5) |
2 (83.7, 121.1) |
304.6 (232.2, 452) |
970.2 (760, 1242.1) |
1526.4 (1141.6, 2590.1) |
DEHP |
|
|
|
|
|
MEHP |
4.0 (3.4, 4.7) |
3.4 (2.8, 3.9) |
9.5 (7, 12.6) |
34.9 (19.6, 48.2) |
62.9 (42.7, 85.8) |
MECPP |
46.8 (39.6, 55.2) |
42.4 (35.2, 50.4) |
126.9 (91.4, 172.2) |
315.9 (238, 454.4) |
612.5 (426.8, 727.5) |
MEHHP |
33.3 (27.7, 40.1) |
30.7 (26.5, 37.2) |
99.6 (71.1, 132.5) |
270.9 (187.1, 417.5) |
486.3 (351.3, 827.3) |
MEOHP |
17.6 (14.8, 20.9) |
15.7 (13.6, 18.8) |
46.7 (36.9, 61.7) |
130.8 (88, 171.6) |
227.5 (152.4, 326.5) |
DnBP |
|
|
|
|
|
MnBP |
77.6 (66.2, 91.0) |
80.1 (67.5, 92.6) |
184.1 (159.2, 217.8) |
383.2 (344.7, 529.4) |
728.9 (492.7, 1032.8) |
DiBP |
|
|
|
|
|
MiBP |
6.2 (5.2, 7.2) |
6.4 (5.2, 7.1) |
15.5 (11.9, 19.7) |
34.5 (26.9, 43.7) |
56.4 (39.3, 74.0) |
BzBP |
|
|
|
|
|
MBzP |
10.3 (8.5, 12.3) |
10.1 (7.9, 12.2) |
31.8 (21.7, 38.6) |
71.6 (55.3, 106.3) |
145.7 (95.6, 242) |
BPA |
2.1 (1.8, 2.5) |
2.2 (1.9, 2.5) |
5.0 (4, 6.5) |
12.4 (9.9, 14.2) |
16.5 (13.8, 21.5) |
Table 2 Average concentrations (in µg/g creatinine) of phthalate metabolites and bisphenol A in 290 urine samples taken from pregnant women in the third trimester of pregnancy in Mexico
Variable |
Factor 1 |
Factor 2 |
Metabolites |
|
|
Ln(MEHHP) |
0.982 |
0.656 |
Ln(MECPP) |
0.976 |
0.636 |
Ln(MEOHP) |
0.973 |
0.638 |
Ln(MEHP) |
0.927 |
0.595 |
|
|
|
Ln(MnBP) |
0.683 |
0.927 |
Ln(MBzP) |
0.505 |
0.841 |
Ln(MiBP) |
0.552 |
0.824 |
Ln(MCPP) |
0.574 |
0.772 |
Ln(BPA) |
0.466 |
0.645 |
Ln(MEP) |
0.339 |
0.535 |
|
|
|
Mean (standard deviation) |
0.000 (0.993) |
0.000 (0.963) |
Median (P25, P75) |
-0.072(-0.678, 0.666) |
0.011(-0.672, 0.618) |
Max, Min |
-2.711, 3.121 |
-2.431, 4.196 |
Table 3 Correlations between rotated common factors and the concentrations of phthalate metabolites and bisphenol A in urine from pregnant Mexican women (n=290)
Structure matrix: correlations between variables and promax(3) rotated common factors. Correlation matrix of the promax(3) rotated common factors was 0.626.
Compared with previously reported exposure indices,24,34–38,45 the two developed in our study (2-ethyl and phthalate mixtures) could be more consistent because they were obtained from the relationships among the compounds, using accurate data, and show its correlation with each other, that is, they are not independent indices.45 Therefore, it could be used as a useful exposure indicator to evaluate the effects on health and it could be used to develop tools to better evaluate exposure to EDCs at life stages when humans are particularly susceptible to their effects. Few studies have investigated prenatal phthalate exposure in Mexican populations. Meeker et al.4 analyzed phthalate metabolites in two groups of women residing in Mexico City (Mexico) who delivered either at-term (≥37 weeks of gestation) or pre-term (<37 weeks of gestation). The phthalate concentrations detected in samples from women with a pre-term delivery were greater than those in women with at-term deliveries were. Compared with the concentrations observed in the present study, the concentrations of three phthalate metabolites in the Meeker study were higher in both groups (MEOHP 18.5 and 25.3µg/g creatinine, MECPP 52.8 and 68.5µg/g creatinine, and MEP 200.0 and 274.0µg/g creatinine for at-term and pre-term deliveries, respectively). In another study carried out in the State of Mexico the metabolites MEHP, MBzP, MnBP, and MEP, showed lower concentrations than those obtained in our study.6 In both earlier studies, the participants had some common sociodemographic characteristics, including their age (average age, 27 years), social class (middle–low), and area of residence (Valley of Mexico). The observed differences in phthalate concentrations may be suggestive of differences in sources, characteristics of participants and lifestyles, despite the demographic similarities. Likewise, the results also suggest that the mixtures of phthalates that individuals are exposed to vary and are different.
To the best of our knowledge, only Cantonwine D et al.46 and Johns LE et al.47 have studied prenatal exposure to BPA. Cantonwine report an average phthalate concentration of 1.95µg/g creatinine and a maximum value of 7.47µg/g creatinine. Johns et al found an average of BPA concentrations of 1.28 at median 10 wk of gestation, concentrations that are below those found by us derived from the fact that the average value found by us was of 2.1µg/g creatinine and the maximum value found by us was more than twice that found by Cantonwine and colleagues.
Compared with the concentrations reported in the 4th Report on Human Exposure to Chemical Substances from the National Health and Nutrition Examination Survey (NHANES) in the U.S,48 which looked at samples from both pregnant and non-pregnant women, the concentrations found in this study for phthalate metabolites were higher by 2.3 to 59.8µg/g creatinine for the geometric means. Only the concentrations of MCPP and MiBP were lower by 0.5 and 2.9µg/g creatinine (geometric means), respectively, in our study than in the NHANES,48 while BPA concentrations were similar. NHANE.48 In another study, Woodruff et al. used a subsample of pregnant and non-pregnant women from the NHANES, and found high concentrations of MBzP, MiBP, MnBP, MEP, and BPA in urine. In this case, the concentrations of MEP, MBzP, and BPA in the pregnant women were higher than the concentrations detected in our study. For MnBP, the results obtained in the present work were higher than those from the NHANES and Woodruff et al. For MiBP, the highest average concentration was detected in the NHANES study (7.5µg/L), while the concentration in our study was slightly lower (7.2µg/L), and lower in the Woodruff study (3.5µg/L). These differences could be caused by several factors such as differences in the populations studied, for example, whether the participants were pregnant or not, differences in exposure for U.S. and Mexican populations, and different phthalate regulation in the U.S.
Other studies around the world have evaluated exposure to these chemicals in prenatal samples, and variations in concentrations have been detected with differences in ethnic background and socioeconomic status. Higher concentrations than those found in our study have been reported in France, where the concentrations of all phthalate metabolites, except MnBP, were up to five times those found in our study.49 In Spain, high concentrations have been detected for MEP, MEHP, MiBP, and MBzP,50 and in Denmark, high concentrations have been detected for MiBP (35.3µg/g creatinine).51 In this study, because of the high correlation between phthalate metabolites and BPA, and because exposure is frequently to a mixture of chemicals, we used the results to establish exposure indices. Other studies have proposed the generation of indices using the sum of the metabolites derived from the principal metabolite,34–36 or from the accumulated risk, generated from the sum of the coefficients between the present level of exposure and the acceptable level of phthalate exposure (the reference value).37 Further studies have looked at using the sum of the molecular weights (MWs) of the metabolites,38 and differentiating between low molecular weight (LMW) and high molecular weight (HMW) metabolites. We compared our results with the following indices proposed by other studies: the DEHP index, which was generated as the sum of the metabolites MEHP, MECPP, MEHHP, and MEOHP; the HMW index, which was generated as the sum of the metabolites MEHP, MECPP, MEHHP, MEOHP, MBzP, and MCPP; the LMW phthalates index, which was generated as the sum of MEP, MnBP and MiBP; and the LMW BPA index, wich was generated as the sum of MEP, MnBP, MiBP, and BPA. We observed highly significant correlations (p < 0.05) between the 2-ethyl index proposed in this study and the DEHP index was (r=99.7%), and the HMW index (r=95.8%). These correlations could be attributed to the fact that these three indices share many of the same metabolites. The only exceptions were the inclusion of MBzP and MCPP in the HMW index. These metabolites are not included in the other two indices because they differ in water solubility to the other metabolites. The second index developed in this study, phthalate mixtures, had a significant correlation of 91.3% (p< 0.05) with the LMW phthalates index, and a significant correlation of 92.7% (p<0.05) with the LMW BPA index.. These comparisons show that our indices take into consideration more than one chemical characteristic, such as molecular weight or water solubility, reflecting the correlation between our indices and the indices proposed by other authors. On one hand, there is a high correlation between the 2-ethyl index with DEHP index and with HMW index, because the metabolites are HMW compounds derived from DEHP. The molecular weight is important to consider because it is a parameter that affects the process of metabolization.
In a study in rats at 7 days post-exposure,52 the amounts of derivatives of phthalate esters excreted in urine and feces decreased as the MWs increased. This may be because the LMW phthalates are rapidly metabolized to their monesters, whereas metabolism of HMW phthalates requires oxidation.53–55 On the other hand, the solubility of a phthalate also determines how long it is retained in an organism (Table 4), and phthalate solubility is known to decrease as the number of carbons in the molecule increases.3,56–58 Phthalates with more carbons are more liposoluble, which can also affect their residence time in an organism. Furthermore, the use of each type of phthalate depends on its physicochemical characteristics, including its MW, the alkyl group to which it is bound, its volatility, and its solubility. From a statistical viewpoint, these groupings are the result of correlations among the metabolites, that is, women who presented with a high concentration of a given metabolite also had a high concentration of another metabolite in the same group but not of metabolites in other groups. In this regard, the exposure indices can be explained by the sources of the phthalates and high scores for the same index should indicate similar exposure sources.
Phtalate parent compound / Bisphenol A |
|
Primary metabolites in human urine |
|
|
|||||||
Name (abbreviation) |
CAS registry number |
Molecur weight |
Partition coefficient |
Water Solubility |
|
Name (abbreviation) |
CAS registry number |
Moleculr weight |
|
Name (abbreviation) |
Moleculr weight |
Di(2-EthylHexyl) Phthalate (DEHP) C6H4[COOCH2CH (C2H5)(CH2)3CH3]2= C24 H38 O4 |
117-81-7 |
390.6 |
7.60 |
0.27 mg/L at 25°C |
|
Mono(2-EthylHexyl) Phthalate (MEHP) |
4376-20-9 |
278.3 |
|
Mono(2-Ethyl-5-CarboxyPentyl) Phthalate (MECPP) |
308.3 |
Mono(2-Ethyl-5-HydroxyHexyl) Phthalate (MEHHP) |
294.3 |
||||||||||
Mono(2-Ethyl-5-Oxohexyl) Phthalate (MEOHP) |
292.3 |
||||||||||
Di(n-Octyl) Phthalate (DnOP) C6H4[COO(CH2)7 CH3]2=C24 H38 O4 |
117-84-0 |
390.6 |
8.10 |
0.022 mg/L at 25°C |
|
Mono(3-CarboxyPropyl) Phthalate (MCPP) |
S/N |
278.3 |
|
|
|
Benzyl Butyl Phthalate (BzBP) CH3(CH2)3OOCC6H4COOCH2C6H5=C19 H20 O4 |
85-68-7 |
312.36 |
|
2.69 mg/L at 25°C |
|
Mono(Benzyl) Phthalate (MBzP) |
2528-16-7 |
256.3 |
|
|
|
Di(n-Butyl) Phthalates (DnBP) C6H4[COO(CH2)3CH3]2=C16 H22 O4 |
84-74-2 |
278.3 |
|
13 mg/L at 25°C |
|
Mono(n-Butyl) Phthalate (MnBP) |
131-70-4 |
222.2 |
|
|
|
Di(iso-Butyl) phthalate (DiBP) C6H4[COOCH2CH(CH3)2]2=C16 H22 O4 |
84-69-5 |
278.3 |
4.11 |
6.2 mg/L at 24°C |
|
Mono(isoButyl) Phthalate (MiBP) |
S/N |
222.2 |
|
|
|
Bisphenol A (BPA) (C6H4 OH)2 [C(CH3)2] =C15 H16 O2 |
80-05-7 |
228.3 |
3.32 |
300 mg/L at 25°C |
|
|
|
|
|
|
|
DiEthyl Phthalate (DEP) C6H4(COOC2H5)2=C12 H14 O4 |
84-66-2 |
222.2 |
2.47 |
1,080 mg/L at 25°C |
|
Mono(Ethyl) Phthalate (MEP) |
2306-33-4 |
194.2 |
|
|
|
Table 4 Physicochemical characteristics of phthalates and their metabolites, well as of the bisphenol A
CAS, chemical abstracts service. S/N, Without number. Molecular weight in g/mol. Partition coefficient: Octanol/water partition coefficient (log Kow)
The 2-ethyl index contains metabolites originating from the metabolism of DEHP, which is mainly consumed as a contaminant in foods that are high in fat, such as milk, fish, or oil.26,59,60 Exposure to DEHP can be primarily attributed to the materials used to package food.61 Additional sources of exposure to DEHP include medical devices, with patients undergoing hemodialysis, neonates, and women having a Cesarean-section delivery being more vulnerable because of the direct contact that they have with these substances in medical equipment.28 In our study, we analyzed samples taken during pregnancy, and analyses were also conducted for the same metabolites in samples taken at delivery in a subsample of 10 women. For these women, there was a correlation, which fluctuated between 10% and 62%, between the concentrations of the phthalate metabolites during pregnancy and at delivery. However, although some studies have shown that DEHP concentrations differ according to delivery type,49,62,63 we couldn't confirm these observations due to our small sample size at the moment of delivery. In our study, the concentrations of the primary metabolite MEHP showed a correlation of 48% when both samples (during pregnancy and at delivery) were taken into consideration, but this was probably because of our small sample size.
Phthalates in the phthalate mixtures index group are used main to provide softness and flexibility to plastics in floor coverings, carpets, paints, glues, industrial solvents, printer inks, vinyl gloves, packaging sealants, bottle top coatings, cans for food,64,65 and cigarette filters.66–68 These phthalates are prohibited in toys, childcare products, and cosmetics.65 Our findings are encouraging because they provide an indication of the potential risk of the toxic effects caused by exposure to this group of EDCs that although, they are known as low risk for the environment, if they constitute a risk to the health of the exposed population derived for their toxicological characteristics. In addition this index contain DEP, which is used in personal care products, which is also found in polluted water sources as well BPA.32,69
A possible limitations of our study are; first: that of not having the measurement of other metabolites of phthalates, this is reflecting in a poor correlation of some metabolites of phthalates as is the case with MEP and BPA and others lesser extent with the second index and second, to have only one measurement of the metabolites during pregnancy, since it is assumed that the temporary variations of the exposure follow the same pattern for the whole pregnancy, however, another measurement was taken in a subsample at the time of delivery and observed good correlation between both measurements for various metabolites. In conclusion, our results showed strong correlations among chemical substances belonging to the same group, and those from the same source and they share toxicological characteristics. Therefore, the exposure indices generated in our study could be used to evaluate the effect of exposure to multiple endocrine disruptors in exposed population and for use in epidemiological studies in environmental health.
We would like to express our very great appreciation to the participants in the project.
This study was supported by the National Council of Sciences and Technology (CONACYT) [Grant numbers 2013-01-202062]. The study protocol was approved by Emory University’s Human Investigations Board (Atlanta, GA, USA), the Ethic Committee of INSP (Cuernavaca, Mexico), and the IMSS General Hospital’s Human Subjects Boards.
The authors declare that there is no conflict of interest.

©2018 Escamilla-Nuñez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.