MOJ
eISSN: 2379-6294


Research Article Volume 3 Issue 4
1Department of Food Toxicology and Contaminants, National Research Center, Egypt
2Department of Food Science Department, Banha University, Egypt
Correspondence: El-Desouky TA, Department of Food Toxicology and Contaminants, National Research Center, Dokki, Cairo, Egypt
Received: June 22, 2017 | Published: July 19, 2017
Citation: El-Desouky TA, Sharoba AMA, El-Desouky AI, et al. Biological and histopathological evaluations of using ozone gas in decontamination of aflatoxinB1 in wheat grains. MOJ Toxicol. 2017;3(4):76–81. DOI: 10.15406/mojt.2017.03.00057
Forty eight Sprague-Dawley male rats were divided into six groups including the control group fed standard diet, the group fed on AflatoxinB1 (AFB1) free wheat treated with ozone gas at 20ppm for 20min mixed with standard diet, the group fed on AFB1 free wheat treated with ozone gas at 40ppm for 20min mixed with standard diet, the group fed AFB1-contaminated diet (2.5mg/kg diet), the group fed on AFB1-contaminated wheat ozonation at 20ppm for 20min mixed with standard diet and the group fed on AFB1-contaminated wheat ozonation at 40ppm for 20min mixed with standard diet. The results indicated that the animals fed AFB1-contaminated diet alone showed a significant decrease in feed intake compared to the control group. Animals fed AFB1-contaminated wheat treated with ozone showed a significant improvement in feed intake and body weight compared to group fed AFB1-contaminated diet. In addition to animals fed AFB1-contaminated diet (2.5mg/kg diet) untreated for 4 weeks showed a significant increase in levels of the biochemical parameters and levels of createnine and uric acid compared to the control group or the groups fed ozone treated wheat alone or the AFB1 -contaminated wheat treated with ozone gas at 20 and 40ppm. On the other hand animal fed on AFB1-contaminated wheat treated with ozone showed a significant improvement in all tested parameters. The Glutathione reduced (GSH) level decreased in animal feed on AFB1-contaminated wheat when compared with the control. Moreover, GSH value in animal feed on contaminated diet after ozonation was related with control group. The histological examination of the liver and kidney tissues showed the animals fed on AFB1 free diet and treated with ozone showed a significant elimination of the harmful effects in the liver and kidney tissues
Keywords: aflatoxin B1, ozonation, wheat, decontamination and biological
A variety of moulds routinely infect the world’s cereal crops. Under certain field or storage conditions, some moulds can produce toxin metabolites “mycotoxins“. Aflatoxins (AFs) are a group of highly carcinogenic mycotoxins produced primarily by the fungus Aspergillus flavus. Within the group of AFs, Aflatoxin B1 (AFB1) have been reported to be carcinogenic, teratogenic, tremorgenic, and dermatitic to a wide range of organisms, and known to cause hepatic carcinoma in humans1 AFB1 is the most potent of the four naturally occurring AFs because of its remarkable Hepatotoxicity and carcinogenicity, this feed contaminant has been the focus of considerable research since its discovery. AFB1 is the most potent carcinogenic substance naturally produced by Aspergillus species. AFB1 is classified by the International Agency of Research on Cancer as Group1 human carcinogen.2 AFB1 has been linked to a specific G to T transversion in the codon 249 in the p53 tumor suppressor gene in primary human hepatocellular carcinoma (HCC).3 The liver is generally the primary target organ and liver damage has been demonstrated in poultry, fish, rodents and non-human primates fed AFB1. Different species have been observed to exhibit different levels of susceptibility to aflatoxins, an example being that in rats and rainbow trout AFB1 is amongst the most potent carcinogens known, but is only either a very weak carcinogen or even non-carcinogenic in guinea pigs. AFB1 have been demonstrated to bind covalently to the N7 of guanine in DNA after P450 mediated activation of the C8-C9 vinyl ether to the highly reactive epoxide. From a toxicological point of view, it is clear that the generation of an 8, 9 epoxide of aflatoxin is an important prelude to the toxicity observed4 Cytochrome P450 enzymes located in the liver are known to convert AFB1 to compounds such as the highly reactive 8, 9 epoxide, aflatoxicol, aflatoxin Q1, aflatoxin P1, and AFM1 depending on the genetic predisposition of the species. Following its formation, the aflatoxin-8, 9-epoxide binds very rapidly to DNA and serum albumin forming aflatoxin-N7-guanine and lysine adducts, respectively.5-7 A variety of chemical, physical, and biological treatments have beentested for their ability to reduce or eliminate the AFs in contaminated feeds and foods. Ozone gas is a powerful oxidant capable of reaction with numerous chemical groups8 Ozone either completely degrades mycotoxins or causes chemical modifications, reducing their biological activity. Ozone reacts across the 8, 9 double bond of the furan ring of AFB1 through electrophilic attack,causing the formation of primary ozonides followed by rearrangement into monozonide derivatives such as aldehydes, ketones and organic acids9,10 The objective of this study was to evaluate the capability of ozone gas to decontamination of AFB1 in contaminated wheat grain and confirm detoxification using laboratory animals
Wheat grains
Wheat grains (Triticum aestivum) were obtained from the South Cairo Mills Company, Cairo, Egypt.
Production of ozone gas
Ozone gas was produced from air using ozone generator Model OZO 6 VTTL OZO Max Ltd, Shefford, QuebecCanada. (OZO MAX LTD, shefford, Quebec, Canada) from purified extra dry oxygen feed gas. The amount of output from ozone was controlled by a monitor- controller having a plug-in sensor on board which is changed for different ranges of ozone concentration and a belt pan in the monitor-controller allows controlling the concentration in a selected range.
Experimental animals
Three-month old Sprague-Dawley male rats (120-130 g) were purchased from the Animal House Colony, National Research Center, Giza, Egypt. The animals were maintained on a standard diet (protein: 16.04%; fat: 3.63%; fiber: 4.1%, and metabolic energy: 2887 Kcal, Kg). After an acclimation period of one week, animals were divided into six groups (8 rats/ group) and housed in filter-top polycarbonate cages housed in a temperature controlled and artificially illuminated room free from any source of chemical contamination
Experimental design
Animals within different treatment groups were treated daily for four weeks as follows:
At the end of the experimentation period (4 weeks), blood samples were collected from all animals from the retro-orbital venous plexus after they had fasted for 12 h for different biochemical analyses. After the collection of blood samples, all animals were sacrificed and the blood samples were collected in a dry clean centrifuge tubes. The tubes were kept for 30 min. to allow blood to clot before centrifugation at 3000 rpm for 10 min. using cooling centrifuge. Serum was separated and stored at - 20 ºC for analysis. Other samples from the livers and kidneys were excised and fixed in 10% neutral formalin for histopathological studies. The tissue samples were dehydrated in ascending grades of ethanol, cleaned in xylene and embedded in paraffin. Sections (8µm) from the investigated organs were cut and stained with hematoxylin and eosin (H&E). For the histochemical investigations Crossman's stain was carried out to demonstrate connective tissue in liver.11
Biochemical assay
The activities of serum alanine transaminase (ALT) and aspartate transaminase (AST) were determined according to.12 The determination of alkaline phosphatase (ALP) in serumwas performed according to the method described by13 Total Antioxidant Capacity level was determined using the enzymatic colorimetric method according to.14 Glutathione was determined in blood according to the methods of.15 Uric acid level was determined using the enzymatic colorimetric method of.16 Creatinine was determined in serum using commercial kits purchased from Biodiagnostic Company., Cairo, Egypt. According to.17 All kits obtained from Biodiagnostic Company., Cairo, Egypt.
Statistical analysis
All data were statistically analyzed using the General Linear Model Procedure of the SPSS var 18. The significance of the differences among treatment groups was determined by Waller-Duncan k-ratio.18 All statements of significance were based on probability of P < 0.05.
The acute toxicity of AFB1 firstly appeared as a significant decrease in feed intake. Animals fed AFB1-contaminated diet alone showed a significant decrease in feed intake compared to the control group or the groups fed ozone treated wheat or the AFB1 -contaminated wheat treated with ozone gas (Figure 1). Our current results in (Figure 2) showed that animals fed AFB1-contaminated diet (2.5mg/kg diet) alone for 4 weeks showed a significant decrease in body weight compared to the control group or the groups fed ozone treated wheat or the AFB1 -contaminated wheat treated with ozone. Results in the indicated that animals fed AFB1-contaminated wheat treated with ozone showed a significant improvement in body weight compared to group fed AFB1-contaminated diet alone.The results indicated that ingestion of AFB1 resulted in a significant decrease in food intake and consequently the body weight gain was also reduced. The reduced feed intake may indicate protein catabolism, thereby contributing to the observed kidney injury and causing impaired glomerular filtration.19 On the other hand, the decrease in body weight in the animals fed AFB1-contaminated diet alone may be due to the effects of AFB1 on the balance between orexigenic and anorexigenic circuits that regulate the homeostatic loop of body weight regulation, leading to cachexia.20 Another mechanism for the reduction of body weight due to AFs ingestion could be that AFs ingestion alters various digestive enzymatic activities that give rise to a malabsorption syndrome, characterized by steatorhea, hypocarotenoidemy, and to lowering of bile, pancreatic lipase, trypsin, and amylase.21 Similar to our results,22 reported that decrease in weight gains caused by the AFs corn was eliminated by treatment of the corn with ozone. Of importance is the fact that performance was not affected by treatment of control corn with ozone. Feed conversion (feed: body weight gain) was not affected by any of the treatments and no mortalities were recorded.

Figure 1 Effect of ozone treated AFB1-contaminated wheat alone or treated with ozone on feed intake in rats.
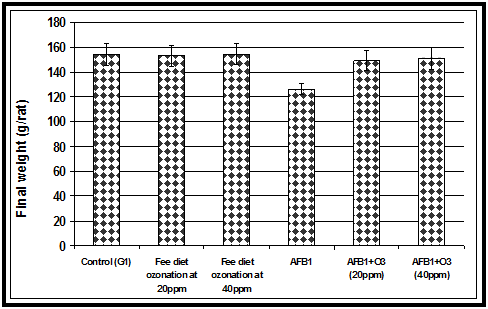
Figure 2 Effect of ozone treated and AFB1-contaminated wheat alone or treated with ozone on final body weight in rats.
Groups |
Parameters (mean±SE)* |
||
ALT (U/L) |
AST (U/L) |
ALP (U/L) |
|
Control |
30.41±0.20a |
56.34±0.21a |
82.35±0.21a |
Fee diet ozone treated at 20ppm |
30.32±0.13a |
56.69±0.06a |
82.65±0.03a |
Fee diet ozone treated at 40ppm |
30.24±0.01a |
56.52±0.18a |
82.39±0.13a |
AFB1 |
48.63±0.01b |
70.29±0.07b |
144.51±0.11b |
AFB1 after ozonation at 20ppm |
31.27±0.02c |
64.29±0.02c |
88.440±0.02c |
AFB1 after ozonation at (40ppm) |
31.16±0.07c |
64.01±0.08c |
88.08±0.27c |
Table 1 Effect of ozone treatment on liver function parameters in rats fed AFB1-contaminated diet (2.5 mg/kg diet)
* Within each column, means superscript with the same letter are not significant different at p ≤ 0.05
The biochemical study
The results of the present study revealed that AFB1 induced severe toxicological effects on serum biochemical parameters tested. The effects of different treatments on ALT, AST and ALP activity in rats are presented in (Table 1). Animals fed AFB1-contaminated diet (2.5mg/kg diet) alone for 4 weeks showed a significant increase in ALT, AST and ALP compared to the control group or the groups fed ozone treated wheat alone or the AFB1 -contaminated wheat treated with ozone gas at 20 and 40 ppm. Analysis of variance indicated that rats fed wheat-contaminated with AFB1 treated with ozone showed a significant improvement in ALT, AST and ALP although these parameters were still higher than the control. The activity of ALT and AST is a sensitive indicator of acute hepatic necrosis, and hepatobiliary disease .23 The increases in ALT, AST and ALP in AFs-treated animals are indicative for changes in the hepatic tissues and biliary system.24 The effect of ozonation on createnine and uric acid was depicted in (Table 2). We observed the significant increase in createnine and uric acid levels in animals fed AFB1-contaminated wheat when compared to the other treated groups. The effects of ozone treatment on Total Antioxidant Capacity (TAC) and GSH are presented in (Table 3). The decrease in (TAC) with AFB1 group might indirectly lead to an increase in oxidative DNA damage.25 The GSH level decreased in animal feed on AFB1-contaminated wheat when compared with the control or the treated groups. Moreover, GSH value in animal feed on contaminated diet after ozonation was related with control group26 reported that GSH, a key antioxidant, is an important constituent of intracellular protective mechanisms against various noxious stimuli, including oxidative stress. GSH depletion in hepatocytes mitochondria has been shown to be an important mechanism in the pathology of experimental liver injury. It has been reported that higher GSH levels help to lower AFB1 toxicity through conjugation with the toxin .27 Several studies on the mechanisms of mycotoxins induced liver injury have demonstrated that glutathione plays an important role in the detoxification of the reactive and toxic metabolites of these mycotoxins, and the liver necrosis begins when the glutathione stores are almost exhausted.28
The histopathological study
The biochemical results were confirmed by the histological examination in liver and kidney tissues. The histological examination of the structure of liver in the control group was normal hepatocytes and central (Figure 3). Liver of rats in group four that fed AFB1-contaminant diet (2.5 mg/kg feed) alone showed enlarged portal area and dilated thick wall portal vein and also appeared accumulation of cellular infiltration and fibrous tissues around proliferated bile ducts thick (arrow) (Figure 4). Rats fed on AFB1 free diet and treated with ozone showed the hepatic cells and central vein are nearly normal (Figure 5). In the other hand, animal in group fed AFB1-contaminated diet after ozonation at 20 and/or40 ppm ozone gas showed the area around the central vein is nearly normal and nearly normal hepatocytes and the damaged area around the portal tracts is not noticed (Figure 6). The liver is the main target organ for AFs and chronic exposure to low levels in foodstuffs cause liver fibrosis and primary liver cancer.29 Microscopic examination of kidney section of control rats showed the normal structure of renal tissue in convoluted tubules and the Bowman capsule (Figure 7). But rats fed AFB1-contaminant diet alon showed distal tubules have fatty degeneration and eosinophilic cytoplasm as well as pyknotic nuclei. Interstatial edema and inflammation also present (Figure 8). While rats fed on AFB1 free diet and treated with ozone showed the nearly normal structural of renal tissue and normal convoluted tubules and the Bowman capsule also rats fed AFB1-contaminated diet after ozonation (Figure 9 & Figure 10). In the current study, animals’ fedAFB1 free diet after ozonation (ozone gas treated wheat alone) did not show any significant effects however, when the AFB1-contaminated wheat grain after ozonation, a significant improvement was observed in the serum biochemical parameters tested and histological examination in liver and kidney tissues. This could be due to the AFB1 been degraded after ozone treatment of the contaminated wheat. These results were in agreement with.22 The primary reaction site of ozone in AFB1 and is the C8-C9 double bond at the terminal furan. This site has been associated with AFB1 toxicity, mutagenicity, and carcinogenic.5 This reaction resulted in the formation of aflatoxinmolozonide which is further change to aflatoxinozonide. This compound is unstable and change to aldehydes, Ketones, acids and CO2 (Figure 11). Ozone is a decomposes to form oxygen gas and therefore can be classified as a no persistent chemical; however, it must be generated at the location of its intended use
Groups |
Creatinine (mg/dl) |
Uric Acid (mg/dl) |
Control |
0.3680±0.006a |
4.19±.039a |
Fee diet ozone treated at 20ppm |
0.3620±0.003a |
4.16±03a |
Fee diet ozone treated at 40ppm |
0.3740±0.005a |
4.17±.02a |
AFB1 |
0.7480±0.006b |
6.31±01b |
AFB1 after ozonation at (20ppm) |
0.4220±0.006c |
4.34±.01c |
AFB1 after ozonation at (40ppm) |
0.4120±0.004c |
4.32±.02c |
Table 2 Effect of ozone treated on kidneysfunction parameters in rats fed AFB1-contaminated diet (2.5 mg/kg diet) (mean±SE)*.
* Within each column, means superscript with the same letter are not significant different at p ≤ 0.05
Groups |
TAC (µmol./g Liver Tissue) |
GSH (mg/dL) |
Control |
104.26 |
0.548 |
Fee diet ozone treated at 20ppm |
104.31 |
0.544 |
Fee diet ozone treated at 40ppm |
104.33 |
0.543 |
AFB1 |
53.72 |
0.067 |
AFB1 after ozonation at (20ppm) |
85.75 |
0.481 |
AFB1 after ozonation at (40ppm) |
85.71 |
0.477 |
Table 3 Effect of Ozone treatment on Total Antioxidant Capacity (TAC) and Glutathione reduced (GSH) in rats fed AFB1-contaminated diet (2.5 mg/kg diet) for 4 weeks

Figure 3 Section in liver of control rats showing the normal histological structure of liver lobule, central vein and hepatocytes separated with blood sinusoids.

Figure 4 Section in liver of rat fed AFB1-contaminated diet showing thickening portal tract with cellular debris and periportal fibrosis. The hepatocytes showing vacuolar degeneration and nuclear pleomorphism.
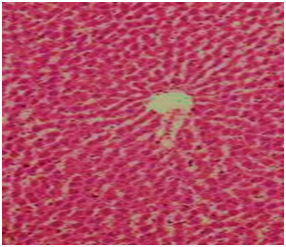
Figure 5 Section in liver of group fed on AFB1 free diet and treated with ozone gas showing the hepatic cells and central vein are nearly.

Figure 6 Section in liver of rat fed AFB1-contaminated diet after ozonation showing significant improvements in liver tissues and normal hepatocytes.

Figure 7 Section in kidney from control rat showing the normal convoluted tubules and the Bowman capsule (H&E200).
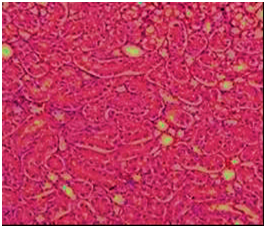
Figure 8 Section in kidney of of rat fed AFB1-contaminated diet showing some distal tubules have fatty degeneration and eosinophilic cytoplasm as well as pyknotic nuclei (arrow) or hyaline casts (circle). Interstitial edema and inflammation also present (H&E 200).

Figure 9 Section in kidney of group fed on AFB1 free diet and treated with ozone gas showing nearly normal convoluted tubules and the Bowman corpuscle (H &E 200).

Figure 10 Section in kidney of rat fed AFB1-contaminated diet after ozonation showing significant improvements in liver tissues and normal hepatocytes (H&E 200).

Figure 11 Mechanism for the addition of ozone to AFs accordting to McKenzie et al.22
Finally from this study, we can conclude that the use of ozone gas to reduction and or removal AFB1 is biologically safe and does not produce any secondary compounds that may cause toxicity. Therefore, ozone gas can be used as one of the most effective and safe methods to removal of AFB1.
None.
The author declares no conflict of interest.

©2017 El-Desouky, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.