MOJ
eISSN: 2379-6294


Research Article Volume 2 Issue 3
1Department of Chemistry, Federal University, Nigeria
2Federal Character Commission, Kano State Office, Nigeria
Correspondence: Dagari MS, Department of Chemistry, Federal University, Nigeria, Tel 8065497722
Received: October 29, 2016 | Published: December 8, 2016
Citation: Dagari MS, Umar GW. Effects of EDTA on uptake accumulation and oxidative stress in spinach (spinacia oleracea l) under copper toxicity. MOJ Toxicol. 2016;2(3):62–65. DOI: 10.15406/mojt.2016.02.00038
The effects of EDTA on Cu2+ uptake by spinach (Spinacea oleracea L) seedlings replanted in hydroponic solutions in a greenhouse investigated. Four week old seedlings, were exposed to various doses of Cu2+ (0, 5, 10, 15, and 20mg/L) and constant concentration of EDTA (10mM). From 15 to 20mg/L Cu2+, there was a substantial increase in copper uptake in Chelated treatments (p<0.05) compared to Unchelated treatments of same concentrations of Cu2+. So, chelation enhanced Cu2+ uptake by spinach. The Cu2+-induced proline accumulation in shoots was also determined. The proline content increased significantly (p<0.05) with increasing Cu2+ concentrations. Free proline is known to accumulate in plants under heavy metals exposure and considered to be involved in stress resistance.
Keywords: proline, hydroponic, chelation, spinach, greenhouse, ethylene diamines tetra acetic acid [EDTA]
Copper is an essential micronutrient in plants that supports many physiological processes including plant growth and metabolism, it plays a significant role in a number of physiological processes such as the photosynthetic and respiratory electron transport chains, nitrogen fixation, protein metabolism, antioxidant activity, cell wall metabolism, hormone perception and as a structural and catalytic component of proteins and enzymes. However, when absorbed in excess quantities, Cu is highly toxic to plant growth potentially leading to physiological disorders Karimi et al.1 It has been reported that excess Cu, at the cellular level, causes molecular damage to plants via the generation of reactive oxygen species (ROS) and free radicals.2,3 Oxidative stress by formation of ROS and oxidation of biomolecules such as lipids, proteins, nucleic acids, carbohydrates, and almost every other organic constituent of the living cell is an important aspect of Cu toxicity. Plant cells can be protected from ROS by enzymatic defense mechanisms like superoxide dismutase (SOD), catalase (CAT), and peroxidase (POD) and nonenzymatic defense mechanisms like free amino acids especially proline, ascorbate, and glutathione and phenolic compounds. Free proline is known to accumulate under heavy metal exposure and considered to be involved in stress resistance Li et al.4 and Karimi et al.1
Growth conditions and treatments
Four week old Spinach (Spinacea oleracea L.) seedlings were carefully collected from Department Agronomy farm, Bayero University, Kano on Wednesday December, 2014 by 4.00pm. They were washed with tap water to remove excess soil, and rinsed three times with deionise water before replanting in hydroponic solution and kept in a greenhouse at 65% relative humidity, 13hrs/day 11hrs/night under 600 μ mol m-2 s-1 of light intensity, and day/night temperatures 39/23°C. Plants were supplied with the Hoagland nutrient solution (pH 6.0-6.3) which contained the following nutrients: 1mM KH2PO4, 2mM MgSO4•4H2O, 5mM KNO3, and 5mM Ca (NO3)2•4H2O and 9µM MnCl2•4H2O, 4.6 µM H3BO3, 0.8 µM ZnSO4•7H2O, 0.3 µM CuSO4•5H2O, and 0.1 µM H2MoO4•H2O. Iron was supplied as Fe-EDTA (1.8 mM). Copper in five levels (0, 5, 10, 15 and 20 ppm) as CuSO4•5H2O were added to the nutrient solution. The concentration of EDTA used was 10mM. Each treatment in triplicates was allowed to stand for five days, after which the plants were harvested and subjected to physiological and biochemical analysis.
Proline content determination
The proline content in the shoots was determined by the method recommended by Bates et al.5
Atomic absorption spectrophotometric determination of Cu2+ in roots and shoots of harvested spinach seedlings
After five days exposure, the spinach seedlings were harvested and washed first with tap water, followed by 1% HNO3 and finally rinsed with deionised water. The roots and shoots were separated and oven dried at 60°C for 48 hours. They were ground with wooden mortar and pestle to a fine powder. A washed dried porcelain crucible was ignited on a hot electric plate for 5minutes. 2g of each sample was accurately weighed into the crucible and gently heated on hot electric plate until the smoking ceased. It was then transferred and ashed to constant weight in a muffle furnace at 5500C for 4hours. The ash was cooled in a dessicator, dissolved in 0.10M HNO3, filtered into 50cm3 volumetric flask and made to mark. The Cu2+ content in the roots and shoots was analyzed using Atomic Absorption Spectrophotometer (Buck Scientific, Model 210VGP) at 324.7nm. The concentration of Cu2+ was reported as mg g-1 dry weight.
Analysis of variance (ANOVA) using the SPSS software was performed to check the accuracy and validity of the results. Data were expressed as mean followed by SD. Statistical significance was assumed at p<0.05.
Figure 1 shows the copper uptake by spinach (Spinacia oleracea L) seedlings grown in Chelated [EDTA 10mM] and Unchelated treatments of same Cu2+ concentrations. There were significant changes in Cu2+ uptake at different concentrations of added Cu2+.This finding is supported by free-ion activity hypothesis which states that “the uptake of trace metals by plants is commonly assumed to depend on the free metal-ion activity, rather than the total concentration of dissolved metal” Degryse et al.6 The Unchelated treatments showed significant variation (p < 0.05) in the uptake of the Cu2+ ion. Between 5 to 10mg/L of added Cu2+ the uptake was 4.79 times higher. However, no much difference was observed between 15 to 20mg/L. These results are in good agreement with the findings of Kumar et al.7 Ozounoudou8 and Karimi et al.1 They generally reported that higher levels of applied Cu2+ enhanced the uptake of the ion by different plants. The changes in translocation factor (TF) and bioaccumulation factor (BF) against the concentrations of added Cu2+ are shown in Figures 2 & 3. According to Chen et al.9 translocation factor (TF) is the ratio of the concentration of a metal in the aerial part of a plant to its concentration in the root.
The effects of TF against concentration of added Cu2+, TF varied significantly with concentration of added Cu2+ (p < 0.05). Thus, a relatively good fraction of the accumulated Cu2+ was translocated to the shoots in Chelated treatments (0.2, 72.7, 32.6, 61.4, and 60.9%) more than the unchelated treatments (0.2, 28.4, 33.8, 61.1 and 33.3%) treatments. The highest values of translocation factor (TF), in Chelated and Unchelated hydroponic treatments were 72.7±0.494% and 54.4±0.0473% respectively. Thus, EDTA aids the translocation of Cu2+ to the shoots. This is supported with the findings of Kralova et al.10 in which EDTA was found to promote the translocation of Cu2+ to the shoots of Chamomile plant (matriacaria recutita L.). The Bioconcentration Factor (BCF) is the ratio of metal concentration in the root to its concentration in the solution.9
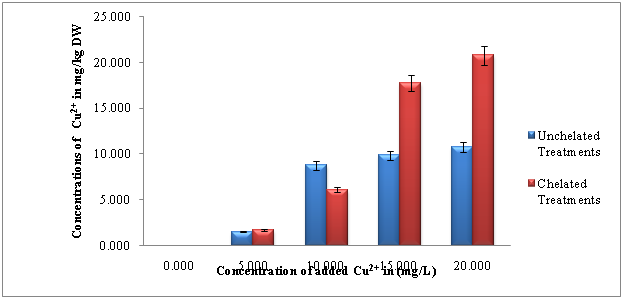
Figure 1 Copper uptake by spinach seedlings (Spinacia oleracea L) shoots in Chelated [EDTA 10mM] and Unchelated Treatments of same Cu2+ Concentrations grown in different concentrations of copper.
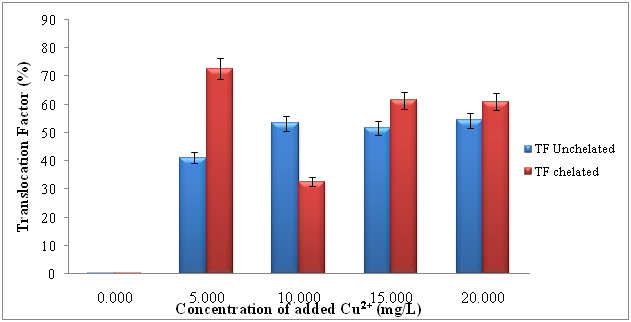
Figure 2 Translocation factor (TF) of Cu2+ in Spinach Seedlings (Spinaceae oleracea L.) grown in Chelated [EDTA 10mM] and Unchelated Treatments of same Cu2+ Concentrations. Vertical bars represent standard error of the mean (n = 3).
The value was used in this research to explain how the Cu2+ ion get closer and cluster around root of spinach of the plant from the solution to be uptake by the root. The BCF showed that the bulk concentrations of Cu2+ remained in the roots. Furthermore, the concentration of Cu2+ in the roots of Chelated hydroponic treatments is more than the unchelated hydroponic treatments. The highest BCF of Chelated hydroponic treatment was 193.0±0.055% and unchelated hydroponic treatment 171.1±0.038%. This result was in agreement with the findings of Chen et al.9 in which the Chelators: EDTA, EDDS and citric acid were found to enhance the Phytoextraction of Cu, Zn, and Pb into high biomass vetiver (Vetiveria zizaniodides). The effects of EDTA were visible between 24 - 48hours of replanting where the leaves appeared damaged and started to wilt. Necrosis also manifested indicating phytotoxicity as shown in plates 3.1, 3.2, 3.3, and 3.4, below To justify the Cu-uptake by the plants, proline was determined to assess the level of stress and damage caused by Cu uptake in Chelated and Unchelated treatments to the plants. Figure 4, shows Cu-induced proline accumulation in shoots. The proline content increased substantially with increasing Cu concentrations (p < 0.05). The proline contents of seedlings in Chelated treatments were relatively higher than those in unchelated treatments of same concentrations of Cu2+ (p < 0.05). At 20mg/L the Chelated treatments showed the highest proline accumulation (3.65±0.014µmol/g) than the unchelated treatments (3.254±0.023µmol/g). This was supported by the highest reduction in dry weight of harvested seedlings (35.90%) compared to other treatments. Similar finding was reported by Karimi et al.11
Application of various doses of Cu2+ (0, 5, 10, 15 and 20mg/L) and 10mM EDTA to hydroponic solutions enhance the Phytoextraction of Cu2+ in spinach (Spinacia oleracea L) seedlings. Varying degrees of phototoxic symptoms which include chlorosis, necrosis and reduction in dry weight of plant were observed depending on the concentration of Cu2+ and presence or absence of EDTA. The highest proline content was found in seedlings grown in hydroponic treated with 20mg/L Cu2+ and 10mM EDTA.
None.
The author declares no conflict of interest.

©2016 Dagari, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.