MOJ
eISSN: 2379-6294


Research Article Volume 2 Issue 1
Research Centre, Riyadh Military Hospital, Saudi Arabia
Correspondence: Md. Wasim Khan, Division of Cell Biology & Physiology, Research Centre, Riyadh Military Hospital, Saudi Arabia, Tel 9197 4852 9884
Received: August 06, 2015 | Published: January 7, 2016
Citation: Wasim KMD, Osman NA, Alahmari AM, et al. Vitamin c protects against viper venom induced hepatotoxicity and oxidative damage in rat liver. MOJ Toxicol. 2016;2(1):1–6. DOI: 10.15406/mojt.2016.02.00026
Snake bite is one of the major causes of death around the globe and a major clinical concern due to improper prognosis as a result of the unavailability of research data. The effect of one sub-lethal dose (4mg/kg body weight) of Bitis arietans crude venom on serum solute/trace element levels and various markers for tissue damage in rat liver have been investigated. Bitis arietans venom caused significant perturbation in the serum levels of electrolytes sodium and potassium and also in the copper: zinc ratio. There was profound hepatotoxicity and nephrotoxicity as evident by increased levels of marker enzymes AST and metabolites creatinine and blood urea nitrogen. This was associated with significant increase in the levels of lipid peroxidation in the liver and concomitant perturbation in the antioxidant enzyme activities. When vitamin C (50mg/kg body weight) was given along with the venom we witnessed a significant ameliorative effect. There was marked improvement in serum levels of hepatic marker enzyme AST and creatinine and urea. Although there was improvement in the levels of copper and zinc upon vitamin C treatment we observed a significant improvement in the sodium: potassium ratio after 24hrs. Vitamin C treatment also significantly reduced the level liver lipid peroxidation and improved the activities of antioxidant enzymes. Taken together our results indicate that viper venom causes oxidative damage to the liver and these effects can be counteracted by the use of an antioxidant.
Keywords: oxidative stress, vitamin c, copper/zinc ratio, snake venom
RPM, revolutions per minute; AST, serum aspartate aminotransferase; ALT, serum glutamate pyruvate transaminase; Scr, serum creatinine; BUN, blood urea nitrogen; C, control; V, venom; VC , vitamin; VVC, venom+vitamin c ; TBS, tris buffered saline; SOD, superoxide dismutase; Cat, catalase; GSH-Px, glutathione peroxidase; LPO, lipid peroxidation
Morbidity and mortality due to snakebite is an important socio medical problem throughout the globe, particularly in the rural areas. The local manifestations caused by viper’s venoms include edema pain, hemorrhage, and necrosis.1-3 Viper’s venom has been implicated in multiple pathologies including neurotoxicity,4 nephrotoxicity,5,6 lung toxicity,7 hepatotoxicity8 and cardiotoxicity.9 Bitis arietans species is responsible for more snakebite fatalities than any other African snake largely owing to its wide distribution, common occurrence, size, potent venom being produced in large amounts and their frequent encounter with humans.10-12 The cytotoxic venom13 is one of the most toxic in the viperidae family. In humans, bites from this species can produce severe local and systemic symptoms. Based on the degree and type of local effect, bites can be divided into two symptomatic categories: those with little or no surface extravasation, and those with hemorrhages evident as ecchymosis, bleeding and swelling. In both cases there is severe pain and tenderness, but in the latter there is widespread superficial or deep necrosis and compartment syndrome.11,12-15 Serious bites results in significant hemorrhage or coagulation in the affected muscles.10-12 The fatality rate highly depends on the severity of the bites and some other factors. Most fatalities are associated with poor clinical management and neglect.11,12
The last two decades have witnessed a major drift in the interests of the scientific community towards providing better means to containing the health risks of the human race. The century old chemotherapies against various disorders have never been a success, albeit not a total failure. Such therapies have a major drawback of side effects that give rise to unseen disorders that emerge as a new challenge. In this regard, the concept of foodstuffs as natural medicines i.e. nutraceuticals has become very attractive. The aim of this study was to investigate the possible beneficial effects of vitamin C in the first line treatment of Bitis arietans venom in rats.
Experimental animals
Male albino Wistar rats weighing 150-200g maintained on a normal chow diet were used. The animals were divided into four groups viz Control (C), Venom (V), Vitamin C alone (VC) and Venom + Vitamin C (VVC) with 8-10 animals in each group. Rats were administered 4.0mg/kg body weight Bitis arietans venom by subcutaneous (sc) injection. One hour after the injection vitamin C (50mg/kg) was administered by oral gavage. The animals were then sacrificed under light ether anesthesia after 3, 6 and 24 hours; blood was collected and preserved till further analysis. The experimental protocol was approved by the Institutional Research and Ethics Committee.
Reagents
All reagents were of analytical grade. Only doubly distilled water was used in preparing solutions, for use as blanks, and for rinsing the equipment. All glass wares were thoroughly cleaned with dilute 0.1M HNO3 and rinsed well.
Venom
The venom used in this study was collected from Bitis arietans snakes, housed and milked by professionals at the Department of Zoology, King Saud University, Riyadh. The fresh venom was filtered and then lyophilized and stored in dark at 4°C. A stock solution of venom (10 mg/mL) was prepared in saline and used in this study.
Serum chemistries
Blood samples were collected at the time of sacrificing the animals and serum was separated by centrifuging at 3000 revolutions per min (RPM) and stored at -20°C until further use. Serum aspartate aminotransferase (AST), serum glutamate pyruvate transaminase (ALT), Serum creatinine (Scr) and blood urea nitrogen (BUN) were determined by commercially available kits from United Diagnostics Industry, Dammam, Saudi Arabia.
Preparation of liver homogenate
The liver samples kept in ice-cold Tris buffered saline (TBS) were homogenized in a glass Teflon homogenizer in 10mM Tris-HCl buffer (pH 7.5) to get 10% (w/v) homogenate followed by high-speed homogenization in an Ultra Turrex Kunkel homogenizer. Aliquots of liver homogenates were divided into three parts. Homogenate was centrifuged at 5000 revolutions per min for 15 min at 4°C and supernatant was used for assay of free-radical scavenging enzymes and the crude homogenate was used for the estimation of total thiol (-SH) and lipid peroxidation.
Atomic absorption standards
In all experiments readymade standards from Perkin Elmer were used. A Perkin Elmer atomic absorbance spectrophotometer (Model A Analyst 800) equipped with an auto-sampler was used in all experiments.
Trace metal determination
Copper and zinc were assessed by the method of Henkin and Meret.16 Sodium and Potassium were determined by the method of Kahn and Fernandez.
Assay of enzymatic and non-enzymatic antioxidants
Superoxide dismutase (SOD) was assayed by the method of Marklund and Marklund.17 Catalase (Cat) and glutathione peroxidase (GSH-Px) activities were determined by the method of Giri et al.18 and Flohe and Gunzler19 respectively. Total -SH was determined by the method of Sedlak and Lindsay20 and lipid peroxidation by the method of Ohkawa et al.4 Protein concentrations in the homogenates were determined the method of Lowry et al.21 as modified by Yusufi et al.22
Statistical analysis
Study groups were compared for variance by one way ANOVA using SPSS 14.0 (SPSS Inc., Chicago, IL, USA). The data were expressed as mean ± SEM; p<0.05 was considered as significant, p<0.01 as highly significant and p<0.001 as extremely significant. Appropriate post-hoc tests were applied were necessary.
Bitis arietans venom causes profound damage to kidney and liver tissues
Venom from the viperidae family of snakes in very well documented to cause severe local damage to tissues especially near the actual site of the snake bite. We used a sub lethal dose of Bitis arietans venom to assess the tissue damaging capability of the venom. We found that the venom causes profound and significant damage to the renal and hepatic tissues after 24 hours of treatment as assessed by the increase in the serum levels of creatinine, blood urea nitrogen and liver enzymes such as AST (Figure 1). In groups where Vitamin C was administered to rats along with the venom we found that there was a significant reduction in the serum levels of blood urea nitrogen and AST. This indicates to a possible protective effect conferred by Vitamin C in snake bite.
Effect of Vitamin C on serum electrolytes Na:K ratio/ serum Copper, Zinc and Cu:Zn ratio after venom administration
The trace-element content of tissues, blood, hair, nails, and excreta of sick and healthy human beings has been studied in relation to a number of diseases (other than nutritional derangements) such as leukemia, multiple sclerosis, diabetes, azotaemia, hepatolenticular degeneration, schizophrenia,23,24 cancer,23-25 liver diseases,24,26,27 parkinsonism,22,24 cystic fibrosis a and others, including cardiovascular diseases, in an attempt to ascertain the existence of a relationship between disease and abnormal trace-element concentration in the human body. In some instances a direct relationship has been found between changes in tissue mineral content and the severity, duration, or incidence of a disease. Although such changes may be secondary to the degeneration and are not a proof of a real cause-effect relation, yet they might afford clues as to the role played by trace elements in the pathogenesis of a given disease; they might also have a predictive value. We have previously shown that the ratio of Na:K and Cu:Zn significantly changes upon venom administration.28 The concentration of serum electrolytes i.e. Sodium (Na) and Potassium (K) was estimated by Atomic absorbance spectrophotometry. We observed that there was no significant change in serum Na at 3hrs and 24hrs post venom administration but a highly significant increase in Na concentration was observed at 6hrs post venom administration (Figure 1). Potassium concentration decreased significantly after 6 hours giving an inverse relation with the concomitant increase in Sodium concentration at the same time. Furthermore, potassium concentration also decreased significantly in serum after 24 hrs of venom administration. The Na:K ratio which signifies renal malfunction increased significantly after 6 hours post venom administration (Figure 2). When vitamin C was administered along with viper venom we observed that it largely affects the levels of potassium in serum (Figure 2). After 24 hours of treatment vitamin C was able to bring the Na:K ratio back close to the normal range (Figure 2).

Figure 1 Effect of venom and vitamin C on serum parameters of hepato/nephrotoxicity.
A-D Rats were treated with venom (4mg/kg) and vitamin C as mentioned in Methods. Blood was collected by heart puncture and serum was collected. All parameters were evaluated with commercially available kits according to manufacturer’s instructions. All panels: n =8-10,*p < 0.05, **p < 0.01, ***p < 0.001compared to Control.
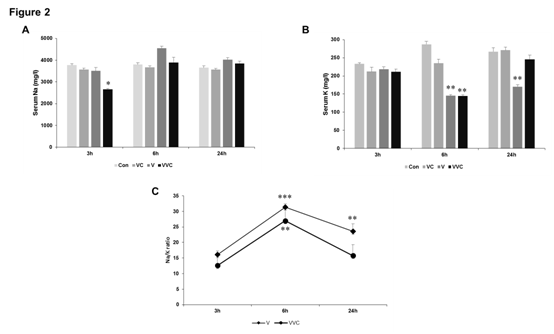
Figure 2 Effect of venom and vitamin C on serum Na and K levels.
A-B Rats were treated with venom (4mg/kg) and vitamin C as mentioned in Methods. Blood was collected by heart puncture and serum was collected. Serum levels of sodium and potassium were analyzed using atomic absorption spectrophotometry as described in Methods. C.
Data from Figure 2A-B was used to calculate the ration of Na:K. All panels: n=8-10, *p < 0.05, **p < 0.01, ***p < 0.001compared to Control.
Serum copper concentrations first tend to decrease at 3hrs and 6hrs post venom administration but then it elevated drastically after 24hrs venom administration. There was no significant change in the levels of zinc in serum post venom administration but there was an extremely significant decrease after 24hrs. The Cu:Zn ratio is considered as a biomarker in the pathophysiology of various types of cancers and other pathologic conditions. We observed an extremely significant increase in the Cu:Zn ratio after 24hrs of venom administration (Figure 3) but the administration of Vitamin C did not affect the rise in the Cu:Zn ratio.
Vitamin C administration protects against hepatic tissue damage induced by viper venom
There is considerable evidence in the scientific literature that snake venom particularly those belonging to the Viper family produces significant amount of tissue damage at the site of bite as well as systemically. To prove whether Bitis arietans induces damage to the liver tissue we checked for lipid peroxidation (LPO) which takes into account the amount of thiobarbituric acid reactive substances formed. Our data indicates that LPO increased with time in rats administered with Bitis venom with significant increase in 24 hours. Interestingly however in rats where Vitamin C was administered along with the venom there was a significant reduction in LPO as compared to the venom group (Figure 4). We could not find any significant changes in the total sulphydryl content of the liver homogenates (Figure 4). Increase in LPO is generally associated with disturbances in the antioxidant enzyme systems so we next thought to analyses the important antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GSH-Px) (Figure 5). Our results indicate that although there was no evident change in the enzyme activities of SOD, there was a profound increase in the activity of CAT whenever Vitamin C was administered along with venom (Figure 5). The activity of GSH-Px showed a marked decrease when venom alone was administered to rats and more importantly Vitamin C was able to significantly increase the specific activity (Figure 5).

Figure 3 Effect of venom and vitamin C on serum Cu and Zn levels.
A-B Rats were treated with venom (4mg/kg) and vitamin C as mentioned in Methods. Blood was collected by heart puncture and serum was collected. Serum levels of copper and zinc were analyzed using atomic absorption spectrophotometry as described in Method C.
Data from Figure 3A-B was used to calculate the ration of Cu:Zn. All panels: n=8-10, *p < 0.05, **p < 0.01, ***p < 0.001compared to Control.

Figure 4 Effect of venom and vitamin C on non-enzymatic antioxidants and LPO.
A-B Rats were treated with venom (4mg/kg) and vitamin C as mentioned in Methods. Liver was collected after sacrifice and homogenized as described in methods. The liver homogenates were used to determine levels of LPO and SH. n=8-10, *p < 0.05, **p < 0.01, ***p < 0.001compared to Control.
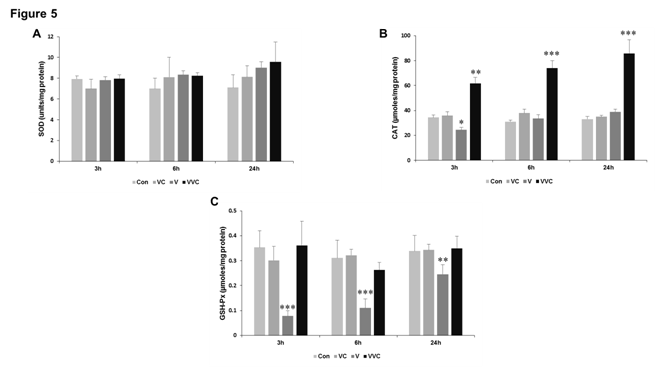
Figure 5 Effect of venom and vitamin C on enzymatic antioxidants.
A-C Rats were treated with venom (4mg/kg) and vitamin C as mentioned in Methods. Liver was collected after sacrifice and homogenized as described in methods. The liver homogenates were used to determine levels antioxidant enzyme activities. n=8-10, *p < 0.05, **p < 0.01, ***p < 0.001compared to Control.
We therefore show that Vipers venom causes tissue damage by increasing free radical mediated damage and that this can be controlled by the administration of antioxidants. This is of profound importance since the first line of intervention that is available in cases of snake bites is anti-venom therapy which itself is harmful to the body. We therefore propose the use of antioxidants along with anti-venom therapy for enhanced clinical care.
None.
The author declares no conflict of interest.

©2016 Wasim, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.