MOJ
eISSN: 2379-6294


Research Article Volume 1 Issue 1
1aap Biomaterials GmbH, Germany
2Justus-Liebig University Giessen, Germany
3Helmholtz-Zentrum Geesthacht, Institute of Material Research, Germany
4TU Berlin Extrusion Research and Development Centre, Germany
5Department of Prosthodontics, Malmo University, Sweden
Correspondence: Olga Charyeva, PhD student, aap Biomaterials, Research and Development, Lagerstrasse 11-15, 64807 Dieburg, Germany,Tel 46735720910
Received: November 20, 2014 | Published: March 3, 2015
Citation: Charyeva O, Feyerabend F, Willumeit R, et al. In vitro resorption of magnesium materials and its effect on surface and surrounding environment. MOJ Toxicol. 2015;1(1):23–28. DOI: 10.15406/mojt.2015.01.00004
Introduction: Magnesium has attracted much attention for its potential use in trauma and orthopedics fields due to its mechanical properties, biocompatibility, biodegradability and the possible ability to stimulate bone formation. It is desirable for magnesium-based alloys to have a low toxicity rate so the fractured bone heals before the implant resorbs.
Materials and methods: Corrosion properties of Mg2Ag, Mg10Gd, WE43 and 99.99% pure Mg were studied under physiological conditions. The samples were placed in DMEM containing 10% fetal bovine serum (FBS) and corrosion was studied after immersion and by gas evolution tests. The corrosion rate (CR), osmolality, pH and Ca2+ concentrations, as well as surface changes in the form of average surface roughness (Sa), developed surface area ratio (Sdr) and summit density (Sds), were determined.
Results: WE43 showed the highest CR of all the materials tested-1.057 mm/year, which is almost twice as high as in the other samples. The lowest mean CR was in the Mg2Ag group. All alloys made pH more alkaline and decreased the concentration of free Ca2+ in the solution. Osmolality decreased in all samples after day 7. Pure Mg had the most constant Sa and Sdr while WE43 had the most stable Sds of all materials over the observation period.
Conclusion: The surface of alloys changed as the implants corroded: the summits became lower with time, while the pitting corrosion progressed. Mg2Ag was the most promising of all the studied materials with regard to toxicity.
Keywords: resorption, biodegradability, magnesium implants, surface
FBS, fetal bovine serum; CR, corrosion rate; DMEM, dulbecco’s modified eagle’s medium
Until recently, the task of modern biomaterial science was to develop stainless corrosion-resistant alloys.1 However, the risk of postoperative infection associated with fixture removal,2 as well as increased incidence of pediatric bone fracture traumas3,4 raised the need for the development of temporary metal implants that would resorb after the healing is complete. Such implants would not only decrease the number of postoperative infections, but also minimize recovery times as well as all associated hospital costs. Magnesium has attracted much attention for its potential use in the trauma and orthopedics fields due to its mechanical properties5 biocompatibility,6 biodegradability and ability to stimulate new bone formation.7,8 Moreover, the inflammatory reaction to magnesium is relatively mild and the alloys show no known allergenic potential.9,10 It is desirable for magnesium-based alloys to have a slow degradation rate so the fractured bone heals before the implant resorbs. It is thus crucial to design alloys with a slow corrosion rate, low toxicity and high biocompatibility. Hard-tissue repair typically requires implantation of the fixture for a minimum of 12weeks.11 During contact with water, magnesium hydroxide accumulates on the surface of the magnesium implant to form a protective corrosion layer, which is also known as a degradation layer.12 As material degrades, various ions are released into solution increasing its osmolality. Corrosion also makes the pH of the surrounding environment more basic, which might influence the normal tissue-healing process.12 The highest clinical knowledge was gained by using supplied or modified WE43 alloys.13–16 Therefore this alloy was chosen as reference. The influence of cells and the in vivo environment on magnesium corrosion is not well described either. It is known that corrosion is faster in vitro than in vivo by several orders of magnitude.10 This can be explained by the presence of proteins and other organic molecules in blood that create a protective coating around magnesium, slowing down corrosion.10 Thus, an addition of proteins in the form of fetal bovine serum (FBS) into the cell growth media would more closely imitate the in vivo environment than just using pure media. Dulbecco’s Modified Eagle’s Medium (DMEM) contains inorganic salts, calcium, amino acids and vitamins, and is thus very close to physiologic conditions.10
Corrosion of magnesium implants also has an effect on a material’s surface characteristics. Surface plays an important role in cell attachment. Both too rough and too smooth surfaces are not beneficial and hinder bone formation around implants.17 Several parameters can describe implant surface topography, such as average surface roughness (Sa), developed surface area ratio (Sdr) and summit density (Sds). Sa is defined as the arithmetic mean of the departures of the roughness area from the mean plane.17 Sdr is the ratio between the 3-D measurement and a 2-D reference plane.17 Sds is the number of summits per unit area making up the surface.18 Parameters describing spatial properties, like Sds, as well as hybrid properties, like Sdr, might further differentiate surfaces with similar Sa characteristics.18 It was shown by previous studies that an optimal Sa value, representing average surface roughness, lies between 1 and 1.5μm for titanium implants.17 However, a positive effect on the bone response of ~ 0.5μm up to ~ 8.5μm was also seen for Sa.19 The long-term corrosion effect on surface roughness of magnesium-based alloys has not been studied yet. The aim of this study was to compare the corrosion of four magnesium-based materials and to analyze their effect on osmolality, Ca2+ concentration, pH and surface changes.
Sample production
The following materials were used to produce alloys for this study: magnesium (99.99%, Xinxiang Jiuli Magnesium Co. Ltd., China), yttrium (99.95%, Grirem Advanced Materials Co. Ltd., China), gadolinium (99.95%, Grirem Advanced Materials Co. Ltd., China), rare earth mixture (Grirem Advanced Materials Co. Ltd., China) and silver (99.99%, ESG Edelmetall-Handel GmbH & Co. KG, Germany). Three magnesium-based materials were produced: Mg2Ag (1.89% Ag, the rest was Mg), Mg10Gd (8.4% Gd, the rest was Mg) and WE43 (3.45% Y, 2.03% Nd, 0.84% Ce, the rest was Mg). Pure magnesium (99.97% Mg) was used as a control. The concentration of magnesium Mg, Y, Nd and Ce was determined by spark emission spectrometer (Spectrolab M, Spektro, Germany) and the concentration of Ag and Gd was determined by X-ray fluorescence spectrometer (Bruker AXS S4 Explorer, Bruker AXS GmbH, Germany). The materials were cast at HZG-MagIC.
The three magnesium alloys (Mg2Ag, Mg10Gd, WE43) were produced by permanent mould gravity casting. After melting the pure Mg, the melt was held at 720°C and the preheated alloying elements were added with continuous stirring for 15 minutes. The melt was poured into a preheated (550°C) permanent steel mould treated with boron nitride. During the casting process, cover gas was used (SF6 and Ar mixture). The alloys were homogenized with a T4 heat treatment prior to extrusion in Ar atmosphere at 550°C (Mg10Gd and WE43) and at 420°C (Mg2Ag) for 6h. Afterwards the alloys were extruded indirectly with an extrusion ratio of 4:25. The chamber of the extrusion machine was set to 370°C and the billets (d=30mm) were preheated for one hour at 370°C (Mg2Ag), at 390°C (WE43) and at 430°C (Mg10Gd). The extrusion speed was between 3 and 4.5mm/sec. Pure Mg was cast by permanent mould direct chill casting.20 The cast billet (d=110mm) was extruded indirectly with an extrusion ratio of 1:84. The billet temperature was 340°C and the speed of the extrusion was 0.7mm/sec. Discs (10mm diameter and 1.5mm thickness) were machined from the extruded bars.
Sample sterilization
The samples were sonicated for 20min in dry isopropanol, dried and gamma-sterilized at the BBF Sterilization Service GmbH facility (Kernen, Germany) with a total dosage of 29kGy.
Corrosion measurements
Corrosion measurements were carried out using two methods: immersion test and hydrogen gas evolution test. Per 0.2g of sample, 3mL of medium consisting of DMEM (DMEM, Life Technologies) with 10% FBS (PAA Laboratories, Linz, Austria) was used. In total, six samples per time point were analyzed. Incubation was performed at 37°C, 5% CO2 and 95% humidity in an incubator (Heraeus BBD 6620, Thermo Fisher Scientific, Schwerte, Germany); oxygen content was set to 20%. The exposure time of the samples was up to 240 hours with medium change every 48 hours. After immersion, the corrosion products were removed by chromic acid (180g/L in distilled water, VWR International, Darmstadt, Germany) at room temperature. The average
Corrosion rate was calculated using the formula:
Where Δg is the weight change in grams, A is the surface area in cm2, t is the immersion time in hours, and ρ is the alloy’s density in g cm-3. The experimental setup for the gas evolution method is depicted in Figure 1. All samples were first weighed and then immersed in DMEM containing 10% FBS. Gas production was measured by eudiometer (400ml, Rettberg, Germany) at room temperature and atmospheric conditions. The graded cylinders were filled with distilled water. The reading was taken every 24 hours. The observation time was 96 hours.
Determination of osmolality and pH
The samples were immersed into DMEM with 10% FBS with medium change every 48 hours. At established time points the medium from twelve wells per group was collected and analyzed. Osmolality was measured with an osmometer (Osmomat 030, Gonotec, Berlin, Germany) and pH measurements were taken using a pH meter (Titan X, Fisher Scientific GmbH, Schwerte, Germany) for each time point.
Determination of Ca2+ concentration
The concentration of Ca2+ in the solution was measured using a calcium analyzer (9180 Electrolyte Analyzer, Roche, Mannheim, Germany) after immersion of the tested alloys into DMEM containing 10% FBS with medium change every 48 hours. In total, the medium from twelve wells per group was tested.
Surface characterization
The samples were immersed into DMEM with 10% FBS and left to corrode in an incubator at 37°C, 5% CO2 and 95% humidity for 3, 5, 7 and 10 days. The medium was changed every second day. At different time points the discs were removed and left to dry at room temperature. Surface characterization was performed with an atomic force microscope (AFM) (XE-100, Park Systems Corp., Suwon, Korea). Measurement areas of 10×10µm in three random positions were selected for each disc. The measurements were performed at a
scan rate of 0.50Hz. The images acquired from the AFM were subjected to leveling and applied Gaussian filtering with a cutoff of 2.5µm using the software Mountains Map® Universal 6.2 (Digital Surf, Besançon, France) and 3-D parameters such as Sa, Sdr and Sds were analyzed. In total, nine surfaces per material group were studied.
Statistical analysis
Data were analyzed using the Statistical Package for the Social Sciences (SPSS, v18, SPSS Inc., Chicago, USA). The significance level was set at 5%. Standard analyses comparing more than two treatments were conducted via one-way repeated measures analysis of variance (ANOVA). One-way repeated measures ANOVA was performed with the Dunn or Holm-Sidak post-hoc test. Surface characterization values (Sa, Sds, Sdr) had non-normal distribution and the Kruskal-Wallis test was performed. The graphs were plotted with SPSS and Microsoft Excel® computer software (MS Excel 2003, Washington, USA).
Corrosion
The WE43 alloy showed the fastest degradation of all the materials measured both by immersion and gas evolution methods, followed by Mg10Gd (Figure 2A). The corrosion of Mg2Ag and pure Mg was comparable, but it was somewhat lower for Mg2Ag. (Figure 2B) illustrates the mass loss at different time points during degradation under cell culture conditions. Gas evolution testing has shown that corrosion tends to slow down after day 3 for all but WE43 alloy (Figure 2C). WE43 was corroding fast in vitro and its degradation did not slow down even after four days. The mass loss graph Figure 2B and the graph showing gas emission Figure 2C have quite similar patterns. The mean corrosion rate was lowest in Mg2Ag, but it was not significantly different compared to WE43 (p=0.09).
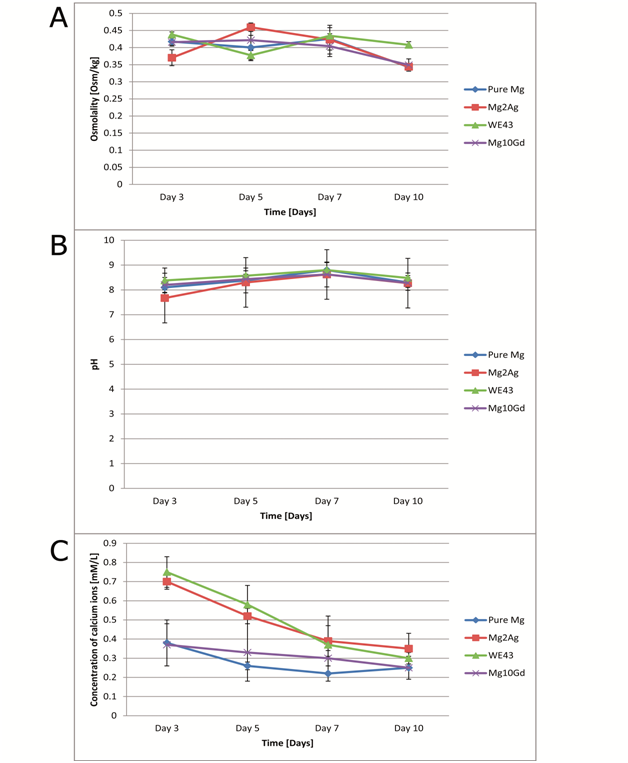
Osmolality
For pure Mg and Mg10Gd, osmolality was generally at a constant level up to day 7 (Figure 3A). After day 7, osmolality dropped for all the tested materials. For Mg2Ag, osmolality had already decreased after day 5. WE43’s osmolality values varied but the lowest osmolality value was reached at day 10.
pH measurements
The original control pH of the medium was 7.9. It was observed that the pH of the medium in which the samples were immersed fluctuated over the observation period (Figure 3B). Generally, pH increased starting from day 3 and reaching its peak at day 7. After day 7 it decreased for all groups. The highest mean pH was for WE43, whereas the lowest mean pH was for Mg2Ag at all time points. No significant differences were found between the groups at various observation periods.
Ca2+ concentration
The concentration of Ca2+ ions in the original control solution was 1.1mM/L. It was observed that Ca2+ concentration generally decreased for all samples (Figure 3C). This decrease was fastest for Mg2Ag and WE43, whereas for pure Mg and Mg10Gd it was more uniform. No correlation was found between Ca2+ and pH of the solution.
Surface characterization
The AFM measurements have revealed that Sa values of Mg2Ag, WE43 and Mg10Gd formed a similar pattern (Figure 4). For these groups, Sa was lowest at day 0 but increased from day 0 to day 3, reaching a peak at day 3. Afterwards Sa decreased at day 5 but then started to increase slowly up to day 10, but this fluctuation was not statistically significant. In contrast, Sa values of pure Mg were quite constant, with significantly higher Sa at day 10 than at day 3 (p<0.001) within the same group. Sdr values of Mg2Ag, WE43 and Mg10Gd showed a similar pattern as Sa for the same materials (Figure 5). The values reached a maximum at day 3, then decreased at day 5 and then started to grow slightly up to day 10. The increase in Sdr values from day 5 to day 10 was not found to be statistically significant. Sdr and Sa of pure Mg had a similar constant pattern, with significantly higher Sdr at day 10 than at day 3 (p<0.001) inside the group.
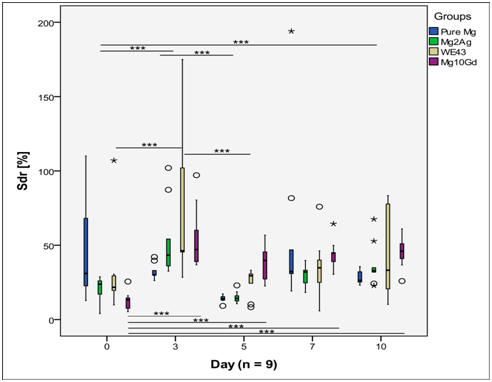
Sds of WE43 was quite constant over the observation period and no statistical differences were found between different observation points (Figure 6). For the other groups, Sds fluctuated and statistical differences between certain time points were found to be significant. Pure Mg and Mg2Ag had a similar pattern of surface change over time. For both of these materials, Sds decreased and reached its minimum at day 5. It then increased again at day 7 but started to fall until day 10, but this decrease was not significant. For Mg10Gd, Sds was minimal at day 3 and maximal at day 5, and then decreased until day 10.
It was shown that WE43 had the highest, whereas Mg2Ag had the lowest, corrosion of all materials. Corrosion rates of Mg10Gd and pure Mg were comparable. It is important that implants that are to be used in medical applications have slow, uniform and thus predictable degradation. Bone healing usually takes around 12 weeks,11 but this can vary on an individual basis depending on patients’ age, general health and medications. Therefore, Mg2Ag, with the lowest corrosion rate, seems to be the most promising of all the tested materials for medical purposes. Our results seem to be consistent with previous studies.20,21 In these studies, WE43 had a higher corrosion rate than pure Mg,20 while pure Mg degraded faster than Mg2Ag.21 Two methods were used to observe corrosion in this study. The immersion test more closely resembles in vivo conditions since it was performed under cell culture condition with constant temperature, oxygen and carbon dioxide. Moreover, medium was constantly changed every second day. The gas evolution test raised a few considerations. Although a eudiometer has previously been used in other studies to measure the gas production of magnesium-based materials such as W4,22 this method seems unreliable. The experiment was performed in a closed system so the gas would not escape, thus the medium was not changed and the access to oxygen was limited. Therefore, the gas evolution method shows corrosion of magnesium-based materials under anaerobic conditions while the immersion method represents corrosion under an aerobic state. In a clinical situation when the implant is placed into the bone the conditions are aerobic, with more or less constant body temperature, oxygen and fluid transportation to and from the healing site. Thus, the immersion test design is closer to a clinical setup while the gas evolution test is very demonstrative of the amount of hydrogen gas that magnesium-based materials produce. Theoretically, osmolality should be high when the corrosion rate is high because degradation products and ions are released from the material. However, this was not the case in this study. Although the corrosion rate of WE43 and Mg2Ag differed by a factor of about 2, there was no statistical difference between the groups. This is because corrosion is a multifactorial process characterized by the release of certain ions (e.g. magnesium and alloying elements) from the alloy to solution and the incorporation of other ions into the degradation layer (e.g. calcium and phosphate). As was shown by earlier studies, Mg2+ facilitates calcification by stimulating the formation of calcium phosphates and thus decreases the amount of free Ca2+ in the surrounding medium.10,20
In fact, all magnesium-based materials decreased the amount of Ca2+ in the solution. Pure Mg and Mg10Gd had a similar effect on Ca2+: first, a high decrease and then only slow further reduction, whereas WE43 and Mg2Ag had a constant rate of depletion. Calcification is beneficial for potential bone implants that are to be used in orthopedic, dental or maxillofacial fields. It was shown in previous research that calcification is promoted at basic pH whereas acidic pH leads to dissolving of calcium crystals.23 In our study, no statistical correlation was found between pH and free Ca2+ ions in the solution. However, it was shown that pH increased and Ca2+ concentration decreased with time for all tested samples. This study showed that magnesium makes pH more basic, and that pH stabilizes after day 7. This stabilization is probably due to the formation of the corrosion layer. The corrosion layer consists of calcium, phosphorous, magnesium and oxygen and is osseoinductive, permeable and corrosion protective.10,20 While high pH is a sign of fast magnesium degradation due to pitting corrosion, lower pH indicates that corrosion is either more uniform or reduced.20 Since magnesium alloys are designed as temporary implants, the fixture will resorb slowly over time, resulting in surface change. This resorption is mainly the result of pitting corrosion.20 This means that the surface of magnesium alloys is not uniform during degradation and is challenging to control. We have seen that Sa and Sdr tend to increase when the alloys are placed into the medium that represents the body conditions. This increase is probably due to pitting corrosion making the surface rougher.
After day 3, both Sa and Sdr decrease, which might be due to the formation of the corrosion layer, which is unstable and tends to break away because the crystalline lattice is easily cleaved.24 This might explain why Sa and Sdr values slightly increase after day 7, although this increase is not significant. Sds tended to decrease, reaching its absolute minimum at day 3 for Mg10Gd and at day 5 for pure Mg and Mg2Ag. This decrease in the number of summits per unit area is due to the cleavage of the surface peaks and irregularities that weaken as corrosion proceeds. For pure Mg, a rapid increase of Sds was observed at day 7 and 10, which might be due to the deposition of calcium phosphate crystals.20 Thus, it can be summarized that when magnesium alloys resorb in vitro, the summits become lower with time, while the pitting corrosion progresses. The formation of the corrosion layer makes the surface more uniform, while the precipitation of calcium phosphates contributes to surface roughness.
Based on the results from this study, it can be hypothesized that the formation of the corrosion layer not only improves conditions in the surrounding environment such as osmolality, pH and Ca2+ concentration, but also makes the surface more uniform. The limitation of this study is that it looked at the corrosion properties of magnesium in the absence of cells. Since these implants are to be used in medical applications, it is desirable to know the effect of cells on corrosion. Further studies examining corrosion properties in the presence of cells are required to see the possible interactions. It is also desirable to compare in vitro corrosion rate with in vivo degradation in future research.
Mg2Ag was the most promising of all the studied materials with regard to corrosion rate and changes to the surrounding environment. Pure Mg had the most constant Sa and Sdr while WE43 had the most stable Sds of all the materials over the observation period. The general pattern of magnesium alloys surface change is as follows: the summits become lower with time, while the pitting corrosion progresses.
None.
The author declares no conflict of interest.

©2015 Charyeva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.