MOJ
eISSN: 2379-6294


Research Article Volume 1 Issue 1
Department of Pharmaceutical Sciences, Northeastern University, USA
Correspondence: Jonghan Kim, Northeastern University, Department of Pharmaceutical Sciences, 360 Huntington Avenue, Boston, MA 02115 USA, Tel 6173 733214, Fax 6173 7388 86
Received: August 11, 2014 | Published: November 26, 2014
Citation: Phattanarudee S, Han M, Kim J. Effect of olfactory manganese dose on motor coordination in iron-deficient rats. MOJ Toxicol. 2014;1(1):1–7. DOI: 10.15406/mojt.2014.01.00001
While exposures to excessive amount of metals increase brain damage, our recent study demonstrated that manganese (Mn) exposure corrected neurobehavioral problems resulting from iron deficiency in young rats. To further characterize the dose-dependent effect of intranasal manganese on motor coordination under iron deficiency, weanling rats were fed iron-deficient (5mg iron/kg diet) or iron-adequate control diet (50mg/kg) for 5 weeks and manganese chloride (MnCl2) solution was intranasal instilled through the right nostril twice a week for 3 weeks. Iron-deficient rats displayed lower blood hematocrit than controls, reflecting an iron-deficient anemic condition. Mn instillation did not alter hematocrit but modestly decreased body weight. In the rotarod test, Mn-instilled rats decreased motor coordination compared with water-instilled control rats (17% decrease in the time of the first drop; P=0.042). Iron-deficiency also decreases rotarod performance. However, upon Mn instillation, iron-deficient rats stayed longer on the bar by 30% than controls (P=0.006). Interestingly, the improvement in motor coordination was associated with manganese dose. Since both iron and Mn support tyrosine hydroxylase (TH), a critical enzyme in dopamine production, we tested if TH expression was modified by Mn instillation under iron deficiency. TH levels in the striatum were increased in iron deficiency and decreased upon Mn instillation, indicating that TH is unrelated with improved motor coordination in response to olfactory Mn under iron deficiency. Taken together, these results demonstrate that either olfactory Mn exposure or iron deficiency decreases motor function but a combination of the two restores poor motor performance by Mn dose-dependent manner.
Keywords: striatum, motor coordination, iron deficiency, olfactory manganese, tyrosine hydroxylase, rotarod
DAT, dopamine transporter; DMT1, divalent metal transporter1; D1R, dopamine receptor 1; D2R, dopamine receptor 2; Fe, iron; ID, iron deficiency; IDA, iron-deficient anemia; Mn, manganese; PND, postnatal day; TH, tyrosine hydroxylase
Iron (Fe) is an essential metal that plays important roles in physiology. Iron is responsible for a vast array of biochemical reactions, including supporting cell division, oxygen transport and mitochondrial function.1,2 Iron deficiency is the most prevalent single nutrient deficiency worldwide2,3 and results in anemia, retarded growth, fatigue, muscle weakness and shortness of breath.2,4,5 Iron is also required in the brain for neurotransmitter synthesis, neurotransmitter signaling, and myelin synthesis of the oligodendrocytes.6,7 Many neurological disorders associated with iron deficiency have been reported. For example, iron deficiency has been associated with poorer fine and gross motor skills, visual-motor integration, language and global IQ, accompanying higher scores in anxiety and depression as well as social and attention problems.8–10
Manganese (Mn), another essential trace element, is also needed for many vital functions, including metabolic processes and anti-oxidant capacities. In particular, manganese superoxide dismutase (Mn SOD) catalyzes dismutation of superoxide anion to hydrogen peroxide and molecular oxygen.11 Manganese is also involved in bone formation and metabolism of amino acids, cholesterol and carbohydrates.12 While Mn deficiency is rarely observed in humans, Mn exposure is significantly associated with neurobehavioral deficits. Manganese neurotoxicity resembles Parkinson’s disease and this has been well-documented in people drinking contaminated water, workers employed in mining and Mn ore processing and agricultural workers exposed to Mn-containing pesticide.13 The use of methylcyclopentadienyl manganese tricarbonyl (MMT), an octane enhancer in gasoline, continues to be a huge concern about potential neurological damage due to inhalable Mn particles after combustion.14 Mn intoxication has also been observed in children under long-term parenteral nutrition and patients with chronic liver diseases.15 Advances in molecular physiology and toxicology have revealed that Mn neurotoxicity is, at least, mediated by dopaminergic dysfunction.16–19 The dopaminergic neurotransmission is also impaired by iron deficiency. There is an accumulating body of evidence indicating that iron deficiency reduces dopamine receptor 1 (D1R) and D2R levels.20–23. In addition, extracellular dopamine level is increased in iron-deficient animals, which is related to a reduced uptake by dopamine transporter (DAT),20,24–26 and returns to normal levels when brain iron levels are corrected.24,26 With respect to Mn, a significant increase in D1R and D2R protein levels was reported in rats exposed to Mn over postnatal day (PND),1–21 particularly in prefrontal cortex and nucleus accumbens.18 Treating adult rats and mice with Mn also increases the number of D2 binding sites in the dorsal striatum.27 In addition, rats exposed to Mn during PND1–21 demonstrated a significant reduction in DAT levels that can persist into adulthood.19,28
An accumulating body of evidence has indicated that Mn absorption is enhanced in iron-deficient anemia (IDA) due to iron-responsive up-regulation of divalent metal transporter-1 (DMT1) which is expressed in intestinal and olfactory epithelium.17,29–31 Our previous study demonstrated that iron deficiency impairs motor coordination, which was corrected by olfactory Mn exposure, suggesting a possibility that Mn could modulate or compensate for some detrimental effects resulting from iron-deficient state.17 However, the dose-dependent effect of Mn to correct the neurobehavioral problem in anemic status is not yet determined. The objective of this study is to determine the dose-dependent effect of olfactory Mn on motor dysfunction found in iron-deficient anemic rats.
Animals and diets
Animal protocols were approved by the Division of Laboratory Animal Medicine (DLAM) and the Northeastern University-Institutional Animal Care and Use Committee (NU-IACUC). Weanling Sprague-Dawley rats were purchased from Charles River Laboratories (Wilmington, MA) and assigned into two groups according to the ranked body weight. Male rats were used because estrogen affects iron metabolism.32,33 Rats were maintained on a 12:12-h light-dark cycle and given water ad libitum provided by DLAM. Rats were fed either iron-deficient diet containing 5mg iron/kg diet (TD.99397, Harlan Teklad, and Madison, WI) or iron-adequate control diet containing 50mg iron/kg diet (TD.07800) for 5 weeks to establish iron-deficient and control cohorts, respectively.
Intranasal instillation of manganese
In order to establish the dose-response relationship of olfactory manganese in iron deficiency, rats fed iron-deficient diet were intranasally instilled with Mn Cl2 (0mg/kg as distilled water, 1, 3, or 10mg/kg body weight; Sigma-Aldrich) at the age of 4-week-old. To determine the interaction effect between olfactory Mn and iron deficiency (i.e., differential effect of intranasal Mn between iron-deficient and control rats), rats fed control diet were also intranasally instilled with either distilled water (0mg/kg) or MnCl2 10mg/kg. Intranasal Mn was administered into the right nostril with thin gel loading tips (0.04mL/kg) under isoflurane anesthesia twice a week for 3 weeks.
Rotarod test
One week after the last dose of intranasal instillation, rats (7.5-week-old) were placed on a standard accelerating rotarod device (Harvard Apparatus). Following 3-day training sessions at fixed speeds (4, 7 and 10rpm on Day 1; 7, 10 and 13rpm on Day 2; 10, 13 and 16rpm on Day 3), rats were tested twice on the rotarod with accelerating speeds from 4 to 40rpm over 5-min (maximum time on bar) or until the rats fell off from the device.17 The rotarod device was cleaned between trials using Quatricide TB (Pharmaceutical Research Laboratories Inc., Waterbury, CT). Time of the first drop was recorded and the better score of the two trials was used for analysis.17
Tissue collection
Rats at the age of 8-week-old were euthanized by isoflurane overdose, followed by exsanguinations and collection of brain. Hematocrit values were determined after blood centrifugation. All tissues were flash-frozen in liquid nitrogen and stored at -80°C until analysis.
Western blot analysis
The right striatum harvested by brain micro dissection was homogenized, electrophoresed on 10% SDS-polyacrylamide gels (40µg proteins) and transferred to nitrocellulose membranes. After blocking with 5% non-fat milk, the membrane was incubated in mouse antibody against tyrosine hydroxylase (1:500; Santa Cruz, Dallas, TX). As a loading control, the immunoblot was incubated with mouse anti-actin (1:5,000, MP Biomedicals, Solon, OH). The blots were incubated with sheep anti-mouse antibody conjugated with HRP (1:1,000, GE Healthcare, Piscataway, NJ), subjected to chemiluminescence (ECL West Dura, Thermo Scientific) and scanned using Chemidoc System (ChemiDoc XRS, Bio-Rad, Hercules, CA). Relative intensities of protein bands normalized to actin were determined using Image Lab (version 4.1; Bio-Rad).
Statistical analysis
Values reported were expressed as means±SEM. A two-way ANOVA was performed using Sigma Plot (version 12.3; Systat Software Inc., San Jose, CA) to determine individual main effects (Mn exposure and iron deficiency) and an interaction effect between iron deficiency and olfactory manganese exposure. A one-way ANOVA was performed to determine dose-dependent Mn effect in iron deficiency, followed by the Holm-Sidakpost-hoc analysis for multiple comparisons. Differences were considered significant at P< 0.05.
Intranasal manganese instillation retards growth in iron-adequate status but not in iron deficiency. Weanling rats fed either iron-adequate control diet (50mg/kg diet) or iron-deficient diet (5mg/kg) received intranasal instillation of manganese chloride (MnCl2) or distilled water (as vehicle control) for 3 weeks (Figure 1) and body weight changes were measured to characterize the influence of olfactory manganese and iron deficiency on growth (Figure 2). All rats studied gained body weight. There was a significant difference in body weight between control and iron-deficient rats (P=0.026, n=7-9 per group) at the age of 6-week-old, but this difference did not persist in later ages (Figure 2A). In contrast, manganese instillation modestly decreased body weight at 6-week-old age (4-11% decrease; P=0.044, n=8-9 per group) and this difference continued up to 8-week-old. These results indicate a negative influence of Mn exposure and iron deficiency on growth in young rats.
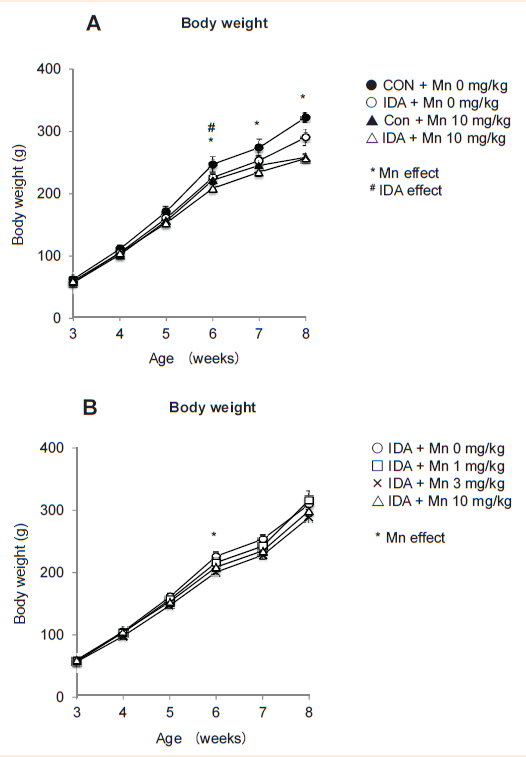
We also tested if olfactory Mn could alter the body weight gain under iron deficiency (Figure 2B). When the rats were 6-week-old, Mn instillation decreased body weight compared with non-exposed iron-deficient rats (Figure 2B). Unlike iron-adequate rats (Figure 2A), however, MnCl2 instillation did not show a significant decrease in body weight in later ages upon iron deficiency (Figure 2B). It might be speculated that Mn could serve as iron for growth when the body is deficient in iron. For example, under iron deficiency some iron-requiring components (e.g., tyrosine hydroxylase) could incorporate and utilize excessive Mn, which otherwise is toxic and inhibits cellular metabolism.17 It is also equally possible that a greater availability of iron transport molecules (e.g., transferring) under iron deficiency34 could capture manganese excess and store/transport as inactive and less toxic form. Biochemical studies designed for iron-manganese interaction would help to identify these potential mechanisms.
Intranasal manganese does not alter hematocrit. Since iron is an essential component for red blood cell production, we tested if manganese exposure could directly or indirectly affect hematocrit values rats in normal and iron-deficiency state (Figure 3). As expected, iron-deficient rats demonstrated significantly decreased hematocrit (Figure 3A) (P=0.002 as overall IDA effect; n=4 per group), while Mn instillation did not modify hematocrit regardless of iron status. Also, there was no dose effect of Mn in iron deficiency (Figure 3B) (P=0.599 as overall Mn effect). Taken together, olfactory Mn exposures impair normal growth, especially when the body iron is sufficient (normal), without altering hematocrit
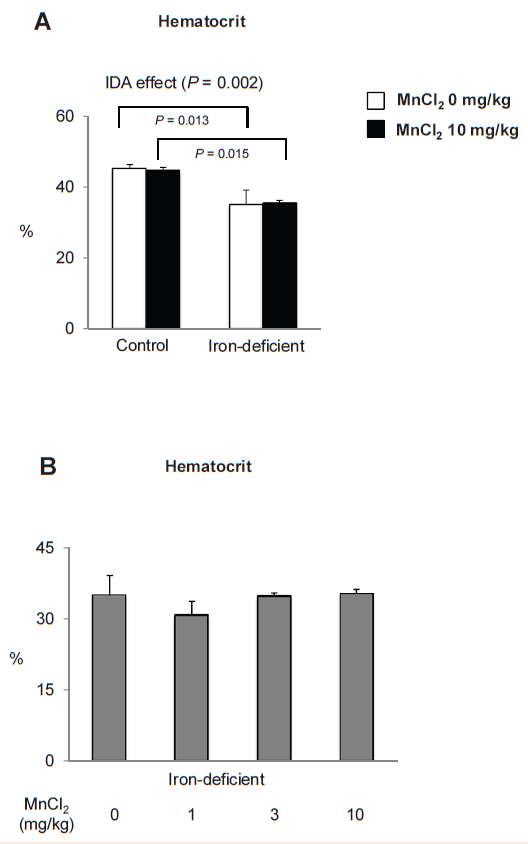
Figure 3 Hematocrit values in iron-deficient and control rats intranasal-instilled with manganese chloride.
Following the rotarod test, rats described in Figure 2 were euthanized and hematocrit values were determined at the age of 8-week-old. Data were presented as mean±SEM (n=4 per group) and analyzed using ANOVA.
Olfactory manganese reverses anemia-induced motor dysfunction by a dose-dependent manner. In order to test if olfactory Mn exposure improves attenuated motor function caused by dietary iron deficiency, we carried out motor coordination test using a standard rotarod device after intranasal instillation of Mn to rats fed control and iron-deficient diets with different doses of MnCl2 (0, 1, 3 and 10mg/kg). There were several important findings (Figure 4). First, Mn-instilled rats fed iron-adequate control diet diminished motor skill by 17%, as evidenced by the shortened time of the first drop from the bar compared with water-instilled rats (Figure 4A) (146±9sec vs 176±11sec, n=8-12 per group; P=0.042). Second, iron-deficient rats instilled with Mn stayed on the bar longer compared with Mn-instilled iron-adequate rats (30% increase; 189±7vs 146±9, n=7-12 per group; P=0.006). This indicates an interaction effect between Mn instillation and iron status (Figure 3A) (IDA x Mn interaction analyzed by two-ANOVA; P=0.008) and further suggests that olfactory Mn administration rescues iron deficiency-associated motor dysfunction, consistent with our previous findings.17 Third, iron-deficient rats exposed to MnCl2 1mg/kg (152sec) or 3mg/kg (161sec) had similar motor impairment to water-instilled iron-deficient rats(160sec), whereas only MnCl2 10mg instillation (189sec) showed a significant increase in motor function by 20% compared with MnCl2 1mg/kg (n=7-11 per group; P=0.031) and exhibited a higher trend (16% increase) compared with water-instilled IDA rats (P=0.102). Combined, these results indicate that intranasal manganese restores motor dysfunction caused by systemic iron deficiency anemia by manganese dose-dependent manner.
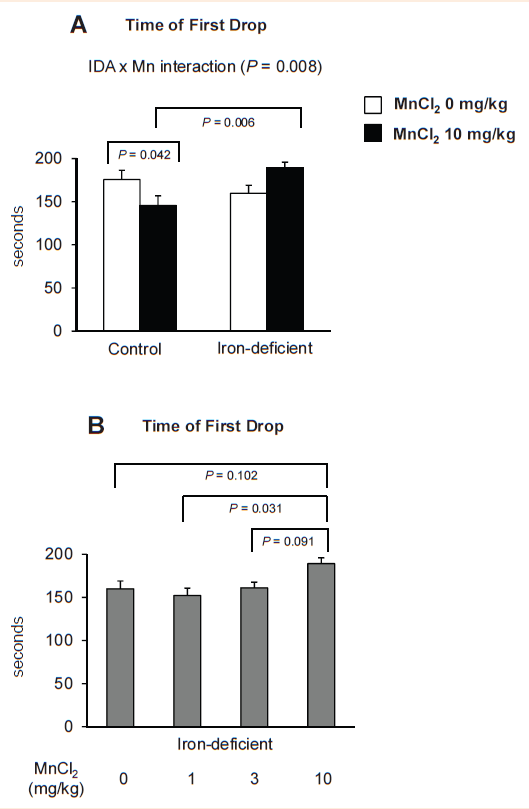
Figure 4 Effect of intranasal manganese instillation and iron deficiency on motor coordination.
Rats intranasal instilled with MnCl2 or distilled water for 3 weeks were tested on the rotarod device to record the time to falling-off. Data were presented as mean±SEM (n=7-12 per group) and analyzed using two-way.
Figure 4A One-way. Figure 4B ANOVA.
Both iron deficiency and manganese overload are associated with motor skill deficits. For instance, in rats, iron-deficiency anemia reduced physical endurance capacity and voluntary use of an activity wheel.35 Glover and Jacobs36 also reported the reduced spontaneous movements in a 24-hr period with iron-deficiency anemia (mild or severe anemia). Similarly, we have recently reported that iron-deficient rats fell sooner from the bar of a rotarod device with decreased speed.17,37 In addition, we have shown that intranasal Mn at the highest dose (MnCl2 10mg/kg) impairs motor function in rats fed normal diet.17 We observed the same pattern in the present study and the results are also in accordance with those reported by Cordova et al.38 who found the impaired motor coordination in the 3rd, 4th and 5th week of life after Mn exposures (10 and 20mg/kg) during PND 8-12. Also, Vezeret et al.39 demonstrated that rats exposed to 15 and 59mg/kg MnCl2 via gavage for 10 weeks showed hypo activity, decreased memory performance and diminished sensori motor reaction. However, our data show that motor coordination is not impaired when these two negative influences (i.e., iron deficiency and manganese loading) were applied together, which is consistent with our previous finding.17 Our results support an idea that Mn exposure could be beneficial for recovering impaired motor coordination found in the iron-deficient state and further suggest that brain dopaminergic neurotransmission could be involved in the modulation of motor performance.17
Tyrosine hydroxylase is altered upon olfactory Mn exposures and iron deficiency. Although motor coordination is controlled by several neurotransmission pathways, the dopaminergic system in the nigrostriatal pathway has been shown to play a predominant role.40 We have demonstrated that amphetamine-evoked extracellular dopamine levels in the striatum were correlated with motor coordination data upon Mn exposure in iron deficiency.17 However, dopamine-related synaptic proteins, including dopamine transporter (DAT), dopamine receptor 1 (D1R) and D2R, were not associated with Mn effect on iron deficiency.17 Therefore, it was hypothesized that the reversing effect of intranasal Mn instillation on motor dysfunction in iron deficiency could result from altered activity of intracellular proteins related to dopamine homeostasis.17 Since tyrosine hydroxylase (TH) is the critical enzyme for dopamine synthesis and since both Fe and Mn can support TH activity,41 we characterized the interaction effect between olfactory Mn exposure and iron deficiency on the expression of TH in the striatum.
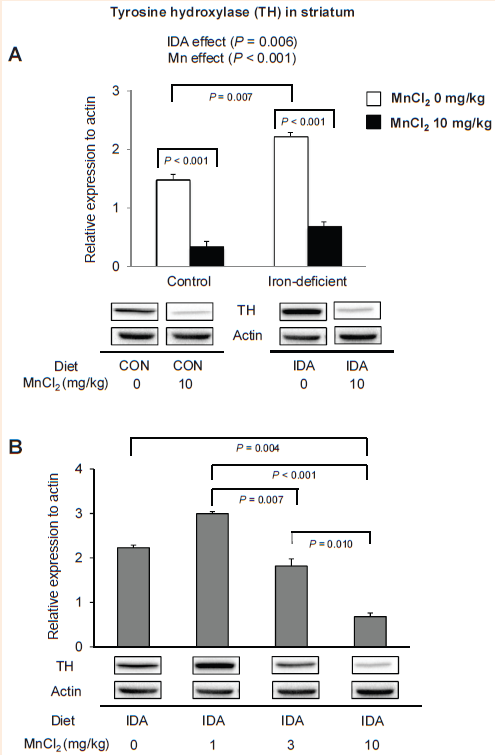
Figure 5 Effect of manganese instillation and iron deficiency on the expression of tyrosine hydroxylase in the striatum. Striatum collected from rats intranasal instilled with MnCl2 was homogenized to determine the expression levels of tyrosine hydroxylase. Relative intensities of protein bands normalized to actin were determined using Image Lab (version 4.1). Data were presented as mean±SEM (n=4 per group) and were analyzed using two-way.
Figure 5A One-way. Figure 5B ANOVA.
We found that both iron and manganese influenced the levels of striatal TH (Figure 5). First, olfactory Mn instillation significantly down-regulated TH by 78% in iron-adequate rats (Figure 5A) (P< 0.001) and by 70% in iron-deficient rats (P<0.001; n=4 per group), which is in agreement with Zhang et al.,42 who showed that chronic manganese exposure(0.1-1µM for 24h) induced a dose-dependent decrease in TH activity of dopaminergic neuronal cell line. Sriram et al.43 also reported that rats exposed to aerosolized welding fumes (manual metal arc-hard surfacing; containing high Mn) of 0.5mg/rat for 7 weeks caused loss of TH protein in the striatum and midbrain. In addition, a sustained loss was observed in the midbrain 105 days after cessation of Mn exposure.43 Second, iron-deficient rats displayed elevated TH levels by 50% increase compared with iron-adequate control rats (Figure 5A) (P=0.007). Upon Mn exposure, this pattern still existed; Mn-exposed iron-deficient rats had 105% greater levels of TH than Mn-instilled iron-adequate rats (Figure 5A; n=4 per group). Our results are in agreement with findings by Unger et al.44 that iron-deficiency beginning on gestational day 15 showed increased TH levels as well as phosphorylated TH levels in prefrontal cortex and striatum at PND 15. However, since iron is required for TH activity, these results do not explain how and why TH protein levels are elevated upon iron deficiency. One possibility is that iron deficiency could reduce TH “activity”, which might subsequently have promoted a compensatory mechanism that up-regulates TH “expression levels”. Third, in contrast to motor coordination results (Figure 4A), there was no interaction effect between Mn and iron deficiency (Figure 5A). Finally, we observed Mn dose-dependent reduction in striatal TH levels in iron deficiency (Figure 5B), which cannot explain the dose-dependent increase in motor performance under IDA status (Figure 4B). These results suggest that changes in TH expression levels are not associated with a beneficial role of manganese in motor coordination.
While we characterized in the present study olfactory uptake of manganese into the brain through the air-brain barrier after intranasal instillation of the metal, manganese transport across the blood-brain barrier (BBB) deserves discussion. Some evidence indicates that both transferring receptor 1 (TfR1) and the divalent metal transporter 1 (DMT1) contribute to Mn transport across the BBB.45,46 Other transporters have been reported to possess an ability to transport Mn. For example, Mn influx across the BBB is partially facilitated by choline transporter.47,48 Also, the transient receptor potential melastatin 7 (TRPM7) has been shown to act as a channel for divalent cation trace metals, including Mn.49 More studies are necessary to understand the exact role of these transporters in the transport of Mn and other metals across the BBB.
Manganese-induced neurotoxicity is most significant when inhaled due to increased olfactory absorption and direct uptake into the brain, providing the relevance to occupational and environmental health and safety.50 Our previous study has provided evidence that impaired motor function due to iron deficiency is rescued by olfactory manganese.17 Thus, in the present study we characterized manganese dose-response relationship under iron deficiency. To our knowledge, the present study is the first to demonstrate the quantitative effect of olfactory Mn exposure on neurobehavioral problems caused by iron-deficient state. Our results demonstrate that either olfactory manganese exposure or iron deficiency reduces motor coordination in rats and that a combination of the two challenges provides a beneficial effect by modulating negative influences of each metal by a manganese-dose dependent manner.
Several possibilities exist to explain dose-dependent effect of olfactory manganese on improved motor skills upon iron deficiency. In the previous and current study, we have excluded the possible influence of TH, DAT, D1R and D2R. It remains to be tested if peripheral muscular activity could have been modified by manganese exposure and iron deficiency since motor performance determined by rotarod device would be controlled by both central and peripheral systems. Altered levels of dopamine and expression of dopamine-related proteins in the cerebellum, another important site for maintenance of posture, balance and motor coordination could contribute to olfactory Mn effect on iron deficiency, although the dopaminergic innervations in the cerebellum is rather low.51,52 It should also be noted that other potential mechanisms could be involved in behaviors related to iron deficiency/manganese overload and that other neurotransmitters could play significant roles in these behaviors. For example, iron deficiency alters serotoninergic53 and GABAergic functions,54 while cholinergic homeostasis is modified by manganese.55 It is also possible that these effects are not due to direct effect by iron and manganese but may be a consequence of other factors such as interactions with other metals, including zinc and copper. Further investigations are warranted to explore these possibilities and to determine the signaling pathways of other neurotransmitters and receptors and their clinical consequences.
None.
The author declares no conflict of interest.

©2014 Phattanarudee, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.