MOJ
eISSN: 2374-6920


Case Report Volume 7 Issue 1
1Department of Transfusion Medicine and Stem Cell Regulation, Juntendo University Graduate School of Medicine, Japan
2Center for Genetic and Chromosomal Analysis, Japan
3Mito-chuo Hospital, Japan
4Department of Hematology, Juntendo University Graduate School of Medicine, Japan
Correspondence: Akimichi Ohsaka, Department of Transfusion Medicine and Stem Cell Regulation, Juntendo University Graduate School of Medicine, 2-1-1 Hongo, Bunkyo-ku, Tokyo 113-8421, Japan, Tel +81 3 5802 1109, Fax +81 3 3811 2724
Received: February 14, 2018 | Published: February 26, 2018
Citation: Kakimoto A, Otsubo K, Saito H, et al. Successful identification of complex rearrangements involving multiple chromosomes in burkitt-type/mature B-cell acte lymphoblastic leukemia: further emphasis on spectral karyotyping . MOJ Proteomics Bioinform. 2018;7(1): 00216. DOI: 10.15406/mojpb.2018.07.00216
We report a case of Burkitt-type/mature B-cell acute lymphoblastic leukemia harboring complex chromosomal rearrangements involving t(8;14)(q24;q32) and IGH/MYC fusion. Multiple chromosome aberrations, where a precise karyotype was not established employing G-banding, were observed at presentation, disappeared in remission, but reappeared on the recurrence of the disease. Spectral karyotyping (SKY) analysis in combination with G-banding revealed eight common aberrations, including t(8;14)(q24;q32). We performed triple-color fluorescence in situ hybridization (FISH) analysis, and identified IGH/MYC fusion signals in 95% of the interphase nuclei analyzed. SKY and FISH analyses may be useful for determining complex karyotypes that were not identified employing conventional cytogenetic alone.
Keywords: burkitt-type leukemia, mature b-cell acute lymphoblastic leukemia, complex karyotype, spectral karyotyping, fluorescence in situ hybridization
Mature B-cell acute lymphoblastic leukemia (ALL) or Burkitt-type ALL is a rare entity and can be defined as the leukemic manifestation of Burkitt lymphoma (BL).1 BL is a highly aggressive B-cell malignancy and can be endemic, sporadic, or associated with immunodeficiency.2,3 Sporadic BL accounts for 1-2% of all adult lymphoma in Western Europe and the United States.2 Mature B-cell ALL is characterized by the expression of pan-/mature B-cell antigens (e.g., HLA-DR, CD19, cyCD22, and CD79a), together with surface immunoglobulin (sIg) accompanying light chain restriction, the association of an L3 morphology according to the FAB classification, and the presence of 8q24/MYC rearrangement.1-3 The MYC gene is most frequently found to be translocated into the Ig heavy chain locus (IGH), resulting in t(8;14)(q24;q32), whereas the less frequently observed variant translocations, t(2;8)(p12;q24) or t(8;22)(q24;q11), juxtapose MYC to the light chain kappa or lambda locus, respectively.1-3 However, there are often discrepancies between the morphology, immunophenotype, and genotype, leading to a heterogenous disease spectrum.
Additional recurrent chromosome aberrations other than t(8;14)(q24;q32) or its variants have been described, with chromosomes 1,6,13,17, and 22 most commonly involved.4 However, unlike acute myeloid leukemia,5 the clinical characteristics of mature B-cell ALL with a complex karyotype (five or more aberrations) remain to be fully elucidated. In this paper, we report a case of mature B-cell ALL harboring complex chromosomal rearrangements, involving t(8;14)(q24;q32) and IGH/MYC fusion, which were successfully identified employing multicolor spectral karyotyping (SKY) and fluorescence in situ hybridization (FISH) analyses.
The patient was a 70-year-old male who presented with flu-like symptoms in April 2010. Hematologic findings upon admission were: hemoglobin (Hb), 8.4 g/dL; platelets, 34x109/L; and white blood cells (WBC), 21.8x109/L with 24% blasts, 1% myelocytes, 4% band-form neutrophils, 14% segmented neutrophils, 1% basophils, 11% monocytes, 45% lymphocytes, and 3 erythroblasts per 100 WBC. Laboratory tests showed 2,356 IU/L lactate dehydrogenase (normal range in our hospital: 106-230). A bone marrow aspirate showed hypercellularity with 93.2% blasts characterized by medium-sized cells with a modest amount of cytoplasm and a few blasts with basophilic cytoplasm and prominent cytoplasmic vacuoles. Immunophenotypic analysis of bone marrow cells at diagnosis by flow cytometry revealed that the blasts were positive for CD5 (57%), CD10 (96%), CD19 (99%), CD20 (96%), CD38 (96%), HLA-DR (100%), and the Ig kappa chain (98%), but negative for CD34, cytoplasmic myeloperoxidase, and cytoplasmic TdT (terminal deoxynucleotidyl transferase), consistent with a mature B-cell phenotype except for CD5 positivity.2 The patient was diagnosed with mature B-cell ALL and treated with the hyper-CVAD plus rituximab regimen,6 the former being modified with a 70% dose. He achieved complete remission (CR) after receiving the first course of the hyper-CVAD phase of the regimen, and was discharged after completing 6 alternating courses of the regimen. In September 2010, the patient was readmitted to the hospital because of recurrence of the disease. A bone marrow aspirate showed normocellularity with 9.8% blasts. The immunophenotype of blasts was positive for CD10 (69%), CD19 (58%), CD38 (98%), and HLA-DR (97%), but negative for CD20 and sIg, suggesting a phenotypic change to precursor B cells. The patient received salvage chemotherapy, but became refractory to it. He died of disease progression and sepsis in January 2011.
The cytogenetic analysis of bone marrow cells at diagnosis by G-banding showed multiple chromosome aberrations, suggestive of a hypodiploid karyotype in 7 out of 10 metaphase cells analyzed (45 chromosomes in 5 cells and 43 chromosomes in 2 cells), and the remaining 3 cells were normal. The tentative karyotype by G-banding was 45,XY,-4,-4,-6,-7,-8,-8,-11,-14,-14,-15,-15,-17,-18,-19,-20,+14mar. However, the complex karyotypes in 7 metaphase cells were not completely identical, and a precise karyotype was not established employing conventional cytogenetics alone. Thus, we performed SKY analysis using a SkyPaintTM kit (Applied Spectral Imaging, Migdal Haemek, Israel), as described previously.7 SKY analysis showed complex rearrangements involving multiple chromosomes (Figure 1). Although SKY analysis was performed on only two cells available, spectral karyotypes were not identical, as in G-banding. The final representative karyotype, combining the results of SKY and G-banding, was 45,XY, der(1)t(1;14)(p13;q13)t(1;17)(q44;q21), -4, der(4)t(4;6) (p11;?), -6,-8, der(11)t(1;11)(?;p15), der(11)t(11;15)(q23;q15), del(13)(q?), der(14)t(1;14)(p13;q13), der(14)t(8;14)(q24;q32),15,der(15)t(11;15)(q13;q11.2),der(17)t(1;17)(?;p13)t(1;15)(?;q15),der(17)t(8;17)(?;p13)t(3;17)(?;q23),-21, der(21)t(3;21)(?;q22),+4mar (Figure 2). The complex chromosome aberrations disappeared in remission but reappeared on the recurrence of the disease. The complex karyotypes at relapse were not completely identical, as at diagnosis. As shown in Table 1, eight common aberrations were identified in each karyotype, as follows: der(1)t(1;14)(p13;q13)t(1;17)(q44;q21),-8, der(11)t(1;11)(?;p15),der(11)t(11;15)(q23;q15),der(14)t(8;14)(q24;q32),der(15)t(11;15)(q13;q11.2), der(17)t(8;17)(?;p13)t(3;17)(?;q23) and der(21)t(3;21)(?;q22).
Chromosomal Aberrations |
4/22/2010 |
5/14/2010 |
6/7/2010 |
9/6/2010 |
9/27/2010 |
12/1/2010 |
der(1)t(1;14)(p13;q13)t(1;17)(q44;q21) |
Y |
N |
N |
Y |
Y |
Y |
-8 |
Y |
N |
N |
Y |
Y |
Y |
der(11)t(1;11)(?;p15) |
Y |
N |
N |
Y |
Y |
Y |
der(11)t(11;15)(q23;q15) |
Y |
N |
N |
Y |
Y |
Y |
der(14)t(8;14)(q24;q32) |
Y |
N |
N |
Y |
Y |
Y |
der(15)t(11;15)(q13;q11.2) |
Y |
N |
N |
Y |
Y |
Y |
der(17)t(8;17)(?;p13)t(3;17)(?;q23) |
Y |
N |
N |
Y |
Y |
Y |
der(21)t(3;21)(?;q22) |
Y |
N |
N |
Y |
Y |
Y |
Others |
*1 |
N |
N |
*2 |
*3 |
*4 |
Table 1 Changes in Chromosomal Aberrations during the Course of the Disease in the Patient
Each karyotype was defined by combining the results of SKY and G-banding analyses (Y: yes; N: no).
*1: –4,der(4)t(4;6)(p11;?),-6,del(13)(q?),der(14)t(1;14)(p13;q13),der(17)t(1;17)(?;p13)t(1;15)(?;q15),-21,+4mar.
*2: add(2)(q21),add(2)(q21),-4,del(13)(q?),der(14)t(1;14)(p13;q13),-15,-16,-17-18,-18,-21,+7mar.
*3: add(2)(q21),-4,-4,add(7)(q22),-13,-13,-14,-16,-17,add(18)(q21),add(19)(q13.1),-21,+8mar.
*4: add(4)(q31),del(13)(q?),der(14)t(1;14)(p13;q13),der(15)t(13;15)(q14;q11.2),t(16;18)(p13.1;q21),der(17)t(1;17)(?;p13)t(1;15)(?;q15),
der(21)t(17;21)(?;p11.2)t(3;17)(q25;?).
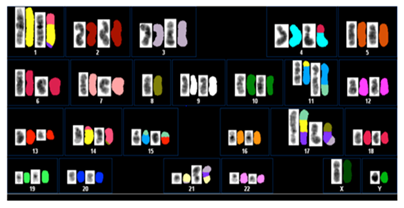
Figure 1 A representative classified spectral karyotype of bone marrow cells at diagnosis showing complex rearrangements involving multiple chromosomes. Reversed DAPI-banded imaging appears on the left of the color display in each box.
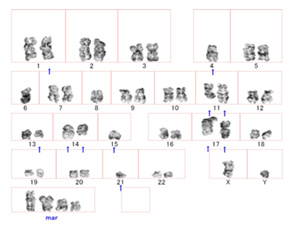
Figure 2 A representative karyotype of bone marrow metaphase cells at diagnosis by combining the results of SKY and G-banding analyses, showing a complex karyotype involving t(8;14)(q24;q32). The arrows represent the derivative chromosome, and the normal chromosome is on the left.
To confirm the presence of t(8;14)(q24;q32) and 11q23 abnormality, the later is related to MLL (Mixed Lineage Leukemia) gene rearrangement, we performed triple-color FISH analysis using the LSI IGH/MYC, CEP 8 Tri-Color, Dual Fusion Translocation Probe and LSI MLL Dual-Color, Break Apart Rearrangement Probe (Abbott Laboratories, Abbott Park, IL, USA), as described previously.8 The expected pattern for a nucleus hybridized with the LSI IGH/MYC, CEP 8 probe in a cell harboring the reciprocal t(8;14) with the 8q24 breakpoint is one orange, one green, two (orange/green) fusions, and two aqua signals. As shown in Figure 3, yellow (orange/green) fusion signals of the IGH and MYC probes were detected in 95% of the interphase nuclei analyzed at diagnosis, indicating the presence of t(8;14)(q24;q32). After achieving CR, the karyotype became normal in all metaphase cells analyzed based on G-banding, and FISH analysis identified no IGH/MYC fusion signals (data not shown). Furthermore, FISH analysis using the LSI MLL probe showed yellow (orange/green) fusion signals of the MLL probe, which were displayed by normal chromosome 11, in 80% of the interphase nuclei analyzed (data not shown), suggesting that the 11q23 region, i.e., t(11;15)(q23;q15), in the patient was not involved in MLL gene rearrangement.
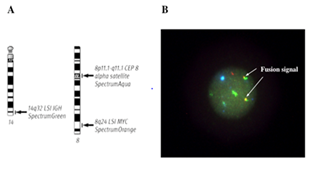
Figure 3 Triple-color FISH analysis of bone marrow interphase cells at diagnosis using probes specific for the IGH and MYC genes. (A) Schematic representation of the FISH probes currently used. (B) Yellow (green/orange) fusion signals of the IGH and MYC probes were detected in 95% of the interphase nuclei analyzed.
The cytogenetic hallmark of BL is t(8;14)(q24;q32) or its variants, t(2;8)(p12;q24) or t(8;22)(q24;q11), resulting in constitutive deregulation of the MYC gene expression driven by the Ig gene enhancer.1-4 Approximately 60 to 70% of sporadic BL cases in adults have additional chromosomal abnormalities, more complex than those found in the uniformly Epstein-Barr virus-positive endemic BL, suggesting potentially more diverse mechanisms of malignant transformation and disease progression in the former.9 When excluding the Burkitt-type translocations, the chromosomes most frequently involved in abnormalities were 1, 6, 13, 17, and 22 (4,9). Boerma et al.10 reviewed the ‘Mitelman Database of Chromosome Aberrations in Cancer’ for defining a cytogenetic profile of ‘true’ BL, where lymphomas were diagnosed on a morphological basis, contained an IG-MYC translocation, and did not harbor chromosomal translocations of the BCL2, BCL6, or cyclin D1 gene (CCND1) loci, and found that additional recurrent abnormalities included gains at chromosome 1q, 7, and 12, and losses of 6q, 13q32-34, and 17p. Poirel et al.11 reported a large cytogenetic study performed on an international trial in children and adolescents with mature B-cell lymphoma, in which the main BL-associated secondary chromosomal aberrations were +1q (29%) and +7q and del(13q)(14% each). The relationship between karyotypic abnormalities and outcomes showed that +7q and del(13q) were independently associated with a significantly inferior event-free survival.10 As for BL with a complex karyotype, Onciu et al.9 reported an analysis for secondary chromosomal abnormalities in sporadic BL comparing pediatric and adult patients, in which approximately half of the patients had a complex karyotype (>3 chromosome abnormalities) in both groups, and the presence of a complex karyotype was associated with a poor prognosis on univariate analysis in children but not in adults.9 In this study, complex karyotypes including t(8;14)(q24;q32) and IGH/MYC fusion were revealed by combining the results of SKY and G-banding. Although the patient achieved CR with the first cycle of the hyper-CVAD plus rituximab regimen, he relapsed with a short CR duration. The short clinical course of the patient may have been, in part, due to the complex chromosomal aberrations.
The characteristic cytogenetic finding of the patient was complex rearrangements involving multiple chromosomes. The complex karyotypes were observed at presentation, disappeared in remission, but reappeared on the recurrence of the disease, being presumably derived from the leukemic clone of the patient. Although the complex karyotypes were not completely identical during the course of the disease, eight common aberrations were identified in each karyotype detected using SKY analysis in combination with G-banding. Of these, the t(8;14)(q24;q32) abnormality may play a central role in the progression of the disease. FISH analysis using probes specific for the IGH and MYC genes revealed the IGH/MYC fusion. Clonal heterogeneity during the course of the disease may result from chromosomal instability.12 Further studies are needed to clarify the issue.
With regard to the immunophenotype of mature B-cell ALL, leukemic cells express sIg and B-cell-specific antigens (i.e., CD19 and CD20), and are negative for CD5, CD23, and TdT.2 They have a germinal center phenotype expressing CD10 and BCL6 but BCL2.2,3 Unexpectedly, leukemic cells of the patient were positive for CD5, which is expressed on all mature T cells and in some B-cell malignancies, such as mantle cell lymphoma (MCL).13 The t(11;14)(q13;q32) is the cytogenetic hallmark of MCL, leading to the over expression of CCND1.14 Although the complex karyotype of the patient included the 11q13 region, which is related to the CCND1 locus, the characteristic t(11;14)(q13;q32) abnormality in MCL was not observed in the patient. Finally, immunohistochemistry of the bone marrow clot section at diagnosis showed CCND1 negativity (data not shown). In addition, the immunophenotype of leukemic cells at relapse was changed into precursor B cells, presumably resulting from the eradication of a mature B-cell clone expressing CD20 by rituximab containing chemotherapy. Interestingly, it has been reported that rare ALL cases with t(8;14)(q24;q32) and an FAB-L3 morphology are associated with a B-precursor immunophenotype.15
In conclusion, we report an elderly patient with mature B-cell ALL harboring a complex karyotype involving t(8;14)(q24;q32) and IGH/MYC fusion. Our case showed the usefulness of SKY and FISH analyses for determining complex rearrangements involving multiple chromosomes that were not identified employing conventional cytogenetics alone.
None.
The authors declare that there are no conflicts of interest regarding the publication of this paper.

©2018 Kakimoto, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.