MOJ
eISSN: 2374-6920


Research Article Volume 5 Issue 6
1Department of Biochemistry and Biotechnology, University of Gujrat, Pakistan
2Department of Biotechnology, University of Gujrat, Pakistan
Correspondence: Muhammad Naveed, Department of Biochemistry and Biotechnology, University of Gujrat, Pakistan, Tel 00923015524624
Received: February 03, 2017 | Published: June 27, 2017
Citation: Naveed M, Zia S, Akhtar M, et al. Molecular docking and pharmacokinetic of highly specific novel pan-mtor inhibitors against solid tumors. MOJ Proteomics Bioinform. 2017;5(6):171-175. DOI: 10.15406/mojpb.2017.05.00177
Mechanistic/mammalian target of rapamycin (mTOR) a serine/threonine kinase belonging to PI3K/Akt/mTOR pathway is involved in different cellular functions cell survival, metabolism, growth, proliferation, apoptosis and autophagy. Pan-mTOR inhibitors are targeted towards mTOR dysregulation, inhibiting the kinase domain of both mTORC1 and mTORC2. The present study analyzes the binding modes and molecular interactions of highly specific mTOR inhibitors, AZD8055 and its sister compoundAZD2014using computational approach. Both inhibitors proved to be effective against solid tumors in vitro and in vivo. Docking analysis was performed using Auto Dock Vina, conformations were scored based upon their binding energy (kcal/mol) and illustrated using Discovery Studio Visualizer 4.5 version. Inhibitors fit between N- and C-lobes of mTOR kinase domain into the inner hydrophobic core. The results indicated interactions with distinctive mTOR residues Trp-2239, Leu-2185 and newly developed interactions with Asp-2375 for AZD2014 and with Ala-2248, His-2247, Thr-2245 for AZD8055. The binding pattern of both inhibitors was slightly different, responsible for better pharmacokinetic profile of AZD2014 and 5 fold increase in efficacy of AZD8055. The binding energy of best docking score for AZD8055 was -8.0kcal/mol however AZD20144 showed best binding affinity at -8.2kcal/mol (RMSD l.b. 0.908, RMSD u.b. 1.075). Highly specific, less toxic and potent inhibitors can be designed or optimized based upon the docking results.
Keywords: in-silico analysis, mTOR, AZD8055, AZD2014, molecular docking, kinase inhibitors, solid tumors
Member of Phosphoinositide 3-kinase (PI3K) family, mechanistic/mammalian target of rapamycin (mTOR) is a serine/threonine kinase and ortholog of yeast TOR protein.1,2 mTOR is a key regulatory enzyme of PI3K/AKT/mTOR pathway and central modulator of cell growth, metabolism and survival.3 mTOR is found in the form of two complexes mTORC1 and mTORC2. Both complexes differ in their function and subunit organization. The mTORC1 nutrient-energy redox sensor, comprises of mTOR, MLST8, PRAS40 and Dept or subunits regulating protein synthesis by phosphorylating eukaryotic translation initiation factor 4E (eIF4E)-binding protein 1 (4E-BP1) and S6 kinase 1 (S6K1). mTORC2 consists of mTOR, MLST8, Rictor, mSIN1 and Protor-1/2 regulating cytoskeleton function, organization and cell survival by phosphorylating AKT, SGK1 and Protein Kinase C alpha.4–6 The domains of mTOR include HEAT repeats at N-terminus, FAT domain, FRB domain, kinase domain and C-terminal FATC domain as shown in Figure 1. Besides being modulator of cell growth, mTOR is a negative regulator of autophagy and hence dysregulation of PI3K/AKT/mTOR pathway results in tumorgenesis.
Different inhibitors are used against mTOR thus blocking the activation of downstream signals; these include 1st generation (allosteric inhibitors rapamycin and rapalogs), 2nd generation inhibitors (ATP-competitive) and new generation inhibitors (Rapalinks and mTORC2 specific inhibitors.7,8 Rapamycin the first known allosteric inhibitor and its derivatives Rapalogs target the FRB domain by forming complex with FKBP-12inhibiting mTORC1 in nano Molar and mTORC2 in micro Molar concentration (Benjamin et al. 2011). Their success has been limited due to hyper activation of AKT by negative feedback loop, mitogen-activated-protein kinase (MAPK) pathway activation through PI3K-dependent feedback loop, less efficient inhibition in case of mTORC2 and because some of the functions of mTORC1 are insensitive to rapamycin inhibition.4,9 ATP-competitive inhibitors that block the proliferation of tumor consist of mTOR/PI3K dual inhibitors (TPdIs) that inhibit kinase domain of mTOR and PI3K, the homolog of mTOR. Such inhibitors have narrow therapeutic range, safety profile, may target other members of PI3K related kinases (PIKK) like DNA-PK in nanomolar concentration and inhibit different isoforms of PI3K like PI3Kα, β, γ and PI3K-δ thus increasing nonspecific inhibition.5,6,10
The key role of involvement of mTOR in multiple cancers led to the discovery of pan-mTOR inhibitors blocking both mTORC1 and mTORC2 with higher specificity over PI3K isoforms.11 Pan-mTOR inhibitors have an IC50 for mTOR inhibition that is significantly lower than that for PI3K.4,12 Pan-mTOR inhibitors ensure inhibition of S6K1 phosphorylation atThr389, complete blocking of S-473 and T-308 phosphorylation of Akt and do not interrupt the negative feedback inhibition of mTORC1, as noticed in case of rapamycin and rapalog mediated inhibition.7,13–16 mTORC2 inhibition mainly effects FOXO signaling pathway and is proved to be less toxic.17 Besides being highly potent against several tumors none of ATP-competitive inhibitors entered clinical phase 3 or available commercially.2,18
AZD2014, rationally designed pan-mTOR inhibitor currently in phase 1 of clinical trials, display higher specificity over PIKK family and exhibit better therapeutic effects against solid tumors.19–21 AZD8055 the parent molecule is no longer in clinical trials and results are expected.22 Owing to the striking role of pan-mTOR inhibitors as effective therapeutic agents extensive study has been done to optimize the lead compounds advanced in clinical trials.23 Developed Torin2 by optimizing Torin1oncogenic target, using focused medicinal chemistry approach.
To identify novel inhibitors analysis of ligand-protein interaction proved to be a valuable tool. The docking results of mTOR/Torin1 and mTOR/Torin2 showed binding of targets at the similar binding pocket (catalytic cleft) but different binding pattern with two additional hydrogen bonds hence 10-fold increase in potency.24,25 Similarly26 provided a list of compounds with higher potency than Evorilumus (rapalog) using molecular docking studies. The present study verifies the in vitro inhibition profile of AZD2014 and AZD8055 and identifies the binding mode and in depth knowledge of the binding pocket in mTOR. To find out the binding positions and molecular interactions of AZD2014 and AZD8055, high score binding modes of both inhibitors were compared to the binding modes of ATP physiological substrate of mTOR reported by Yang et al.,24 which would help in establishing the unique bonding pattern between protein and ligand responsible for higher specificity for mTORC1 and mTORC2 over PI3K isoforms. Two strong hydrogen bonds were reported for AZD2014 with Trp-2239 and Asp-2357 responsible for higher specificity. Weak interaction between AZD8055 and two highly specific residues Val-2240 and Tyr-2225 of mTOR was observed.
Target protein and Ligands
The structures of ATP-competitive inhibitors AZD8055 and AZD2014 were retrieved from (http://www.ncbi.nlm.nih.gov/pccompound) Pub Chem chemical compound database, CID:25262965 and CID:25262792 respectively. The three dimensional structure of mTOR co-complexed with inhibitor PI-103 was obtained from Protein Data Bank (http://www.rcsb.org/pdb/) with PDB ID: 4JT6. The N-terminally truncated mTOR structure bound with natural ligand ATP and co-factors was downloaded from PDB with PDB ID: 4JSP for comparative study of Kinase domain inhibitors.
ATP-competitive inhibitor binding site analysis
Structure of mTOR co-complexed with inhibitor PI-103 was studied using Discovery Studio Visualizer 4.5 version (DS, Accelrys Software Inc., USA). The bound ligand provided information about the ligand binding site atoms, used to identify X, Y and Z grid dimensions.
Inhibitor docking and superimposition of ligands
Before performing docking, the bound ligand and water molecules were removed from mTOR structure using Discovery Studio Visualizer 4.5 version (DS, Accelrys Software Inc., USA). To determine the protein ligand interactions docking analysis was carried out using Auto Dock and MGL Tools v1.5.6 with Lamarckian genetic algorithm.27 Polar hydrogens and Gastieger charges were added to the protein. Grid-Box size was set as X=40, Y=30 and Z=40 with 0.375 Angstrom spacing. Polar hydrogens were added to the ligand and torsions were set as 4. The files of protein and ligand were prepared using MGL Tools and saved in PDBQT format.28 Rigid docking was carried out with all other default parameters. The results of the docking analysis were visualized and illustrated using Discovery Studio Visualizer 4.5 version (DS, Accelrys Software Inc., USA). The binding poses were evaluated on the basis of binding energy in kcal/mol. The low energy binding pose (more stable) of each inhibitor was selected for further study. The resulting AZD8055/mTOR and AZD2014/mTOR complexes were superimposed to ATP/mTOR complex for comparison of binding modes in the mTOR active site using Discovery Studio Visualizer 4.5 version (DS, Accelrys Software Inc., USA).
Docking analysis of AZD 2014
Auto dock vina docking analysis was carried out to study the binding position and molecular interactions between AZD2014 and mTOR. AZD2014 or 3-[2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl]-N-methylbenzamide, analogue of AZD8055 is a novel and highly specific mTOR kinase domain inhibitor present in Phase 2 of clinical trials29 (Figure 2a). All nine binding modes are shown in (Figure 3). We report the best binding pose based upon low binding energy value (-8.2kcal/mol, RMSD l.b. 0.908, RMSD u.b.1.075).Inhibitor fits at V-shaped cleft between N- and C-lobe of kinase domain (Figure 4). The N-methyl benzamide ring and pyrido-pyrimidine group is docked into the inner hydrophobic core; area at the back of the cleft. Two strong hydrogen bonds were established with Asp-2357 and Tyr-2225 (Figure 5). Hydrogen bonding with these residues is a cause of increase in potency as reported by.23,30,31
The N-methyl benzamide group was also engaged in bonding with Ile-2237 and Ile-2356, necessary for kinase domain inhibitors.32–34 The indole group of Trp-2239 interacted with the morpholine ring and stacked with the pyrido-pyrimidine group in a way similar to ATP and Torin2. Trp-2239 contact is responsible for higher specificity over PI3K isoforms2,18,24,35,36 One morpholine ring is surrounded by hydrophobic and the other by hydrophilic residues.37 The side chain of Met-2345part of the N-lobe opposite to adenine binding pocket and Ile-2356 part of hydrophobic adenine site were involved in hydrophobic interactions with pyrido-pyrimidine group. AZD2014 was engaged in bonding with Leu-2185 another residue unique to mTOR.24,38 Additional bond of morpholine moiety with Ile-2163 and Cys-2243 was also observed. Such inhibitory pattern accounts for complete inhibition of mTORC1 and mTORC2 biomarkers by decreasing phosphorylation of 4EPB1 at Thre 37/46 and Akt Ser473, preventing feedback activation of Akt in vitro and in vivo.9,35,39 The 3S-3-methylmorpholin is associated with perfect aqueous solubility of AZD2014.40 Besides interacting with active side residues few amino acids were present in close proximity Ser-2342, Ser-2165, Lys-2187 and non-conventional residues Gly-2238, Val-2240 that attacked the adenine ring in ATP for hydrogen bonding. AZD2014 is rationally designed having IC50 2.8nM and adverse effects similar to other pan-mTOR inhibitors.19
Docking analysis of AZD8055
The (5-{2,4-bis[(3S)-3-methylmorpholin-4-yl]pyrido[2,3-d]pyrimidin-7-yl}-2 methoxyphenyl) methanol or AZD8055 has 0.8nM IC-5020 (Figure 2b). In vitro and in vivo studies showed no pronounced adverse effects and a significant inhibition of downstream signals of mTORC1 and mTORC2 with unexpected tumor sensitivity.11,41 It causes reduction in tumor growth by inhibiting cap-dependent translation and promoting autophagy. AZD8055 inhibits the phosphorylation of S6K atSer235/236 and Akt at Ser473. AZD8055 was proved to be a better therapeutic agent than allosteric inhibitors for endocrine resistant breast cancers.42,43 AZD2014 is 5 fold less effective than AZD8055 but has better pharmacokinetic profile.44 This was proved with docking analysis of AZD8055. Not a single moiety established hydrogen bonding with mTOR residues but weak interactions with two highly specific residues Val-2240 and Tyr-2225 were observed (Figure 6). The binding energy of best docking score was -8.0kcal/mol. Interactions were developed with Trp-2239, Ile-2163, Cys-2243, Ile-2356, Met-2345, Leu-2185 and Ile-2237 similar to AZD2014. However, unique molecular interactions were observed involving Ala-2248, His-2247 and Thr-2245 hydrophilic pocket residues of mTOR. Both morpholine rings interacted with hydrophilic residues. This could be the reason for 5 fold increase in potency (Figure 7).
Comparison of highly specific binding modes
The binding modes of AZD2014, AZD8055 and ATP were superimposed to predict the difference in conformations. The binding energy of best docking score for AZD8055 was -8.0kcal/mol however AZD20144 showed best binding affinity at -8.2kcal/mol (RMSD l.b. 0.908, RMSD u.b. 1.075). The adenine binding site, area of higher hydrophobicity was occupied by N-methyl benzamide ring of AZD2014 and methoxyphenyl group of AZD8055 (Figure 8). The phosphate groups of ATP extend towards the area of lower hydrophobicity and methyl-morpholine moiety of AZD2014 and AZD8055 extend towards the other edge of low hydrophobicity area. Hydrophobic interactions enhance the binding affinity between ligand and protein.30,45 The chemical structure of the inhibitors can be optimized for increasing drug efficacy and specificity based on hydrophobic interactions. AZD2014 shows molecular interactions with more residues however AZD8055 shows interactions with residues unique to mTOR.
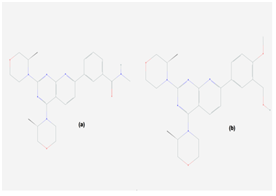
Figure 2Schematic diagram of mTOR domains developed using IBS illustrator.1
The present study verified the in vitro inhibition profile of AZD2014 and AZD8055 to identify the binding mode and in depth knowledge of the binding pocket in mTOR. The docking analysis revealed the molecular interactions common to all pan-mTOR inhibitors and binding pattern unique to AZD2014 and AZD8055.The difference in the binding affinity of both inhibitors is due to the presence of benzamide and phenyl group in AZD2014 and AZD8055 respectively. The molecular interactions could be reevaluated based upon high resolution structure of mTOR. Unpredicted effects of novel interactions can be studied based upon computational approach. From this we predict that the success rate of both inhibitors in clinical trials and docking results could be helpful in evaluating their efficacy against cancers other than solid tumors. Highly specific, less toxic and potent inhibitors can be designed or optimized based upon the docking results.
None.
The author declares no conflict of interest.

©2017 Naveed, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.