MOJ
eISSN: 2374-6920


Research Article Volume 4 Issue 6
1Department of Chemistry, University of the Valley, Colombia
2Department of Chemistry, University of Quindio, Colombia
3Faculty of Health, Universidad del Valle, Colombia
Correspondence: Fabio Zuluaga, Department of Chemistry, University of the Valley, Cali, Colombia
Received: November 02, 2016 | Published: December 29, 2016
Citation: Peñaranda-Armbrecht J, Agudelo C, Zuluaga F, et al. Synthesis of poly (lactic acid) and production of scaffolds by electrospinning. MOJ Proteomics Bioinform. 2016;4(6):334-338. DOI: 10.15406/mojpb.2016.04.00140
In this research, the synthesis of L-lactide (L-Lc), the cyclic dimer of poly (L-lactic acid) (PLLA) with a 55.20% yield and its polymerization to PLLA with different molecular weights is reported. The product was used to produce fibrous scaffolds by electrospinning using variations in concentration, distance, flow and voltage. A high-resolution stereoscope was employed to find fiber diameter (500 y 1000μm); PLLA with 295,000g/mol molecular weight (viscometry) was obtained after 96hours of reaction and yielded the best results in the electrospinning process. These scaffolds were evaluated in vitro for cell growth and cytotoxicity with human skin fibroblasts. None of the samples were cytotoxic above the permitted threshold of 50%. This study provides an important increase in performance for the synthesis of poly (L-lactic acid) (PLLA) and further development in the manufacturing of scaffolds which have multiple uses.
Keywords: Poly (L-lactic acid); L-Lactide; Fibrous scaffolds; Cytotoxicity
ROP, ring opening polymerization; LA, lactic acid; DSC, differential scanning calorimetry; ECM, extracellular matrix; DMEM, modify medium eagle´s dulbeco; FBS, fetal bovine serum; TGA, thermo gravimetric analysis
The possible depletion of non-renewable raw materials and the overall increase in pollution has promoted the search for renewable sources for the production of polymers, as well as the synthesis of biodegradable polymers.1,2 Aliphatic polyesters have emerged for short and medium term applications such as sutures, films, dental materials, bags, containers for beverages and disposable materials, due to their biocompatibility and biodegradability.3 Among these, polylactic acid, PLA, has been the most studied, for several reasons: it comes from a natural source, is biodegradable and its degradation products are absorbed by the body.4 It has also been suitable for creating devices such as porous blocks, films and screws. These bioabsorbable devices have been widely used for orthopedic purposes and they also possess great advantages compared with titanium implants or other metal implants because they do not erode the bone when implanted. Additionally, they do not require an extra surgery for removal, which reduces medical costs and allows the gradual recovery of tissue as the material is degraded. Recent advances include fabrication of stents subjected to surface treatments such as pglation to control protein deposition.5 Most of the applications require the production of high molecular weight PLA which can be achieved using either high boiling solvents or depolymerization of oligomers to obtain the dimer called lactide, followed by Ring Opening Polymerization (ROP). This process has been studied with regard to the influence of many parameters such as temperature, pressure, percentage and nature of the catalyst on the molecular weight of the polymer.6 Several methods have been designed for the production of scaffolds, such as membranes or porous blocks, by solvent evaporation, lyophilization, particulate leaching,7–9 foams by injection and subsequent leaching of carbon dioxide at high pressure,10 porous fabrics by electrospinning,11,12 phase separation,7–9,13 additive manufacturing,14 laser sintering,14,15 and 3D printing.7,13–16 Electrospinning is a technique patented in 1934, by Formhals17 where a polymer solution is pushed through a needle with the aid of an electric field to produce fibers with a diameter ranging from nanometers to micrometers.
This article describes the synthesis of L-lactide (L-Lc), for subsequent ring opening polymerization (ROP) to PLLA with different molecular weights and electrospinning tests to obtain fibrous scaffolds for in vitro cell growth.
Synthesis of low molecular weight PLA (Oligomers)
40mL of L-(+)-lactic acid (LA) (88 to 92%, Scharlau) were placed in a dry 200mL round bottom flask immersed in a sand bath with constant stirring. Starting at 70°C, and 700mbar, temperature and pressure variations were performed every five minutes, continuing with 90°C, 400 mbar, 100°C, 300 mbar, and from 110 to 150°C, 100 mbar. After reaching 150°C, 100 mbar, the process continued until complete water removal to yield 20g of the PLLA oligomer (450-1000g/mol, density 0.825g/mL) following procedures previously established in our laboratory.18
Crude Lc synthesis
The oligomer was distilled at 250°C to 260°C, adding 1.5% SnCl2. 2H2O (Riedel - de Haën ), collecting the crude Lc in a 200mL round bottom flask, dipped in an ice bath, and connected to a condenser with circulating water at 4°C. This procedure supplied 18.74g of crude Lc, with a yield of 55.20%. After recrystallization with ethyl acetate in a 1:2 weight ratio with respect to crude Lc, 15.45g (46.50% yield) were produced.
Synthesis of high molecular weight PLA through ROP
2g of L-Lc, 13.84µL of Sn(Oct)2 (Alfa Aesar), and 220.04mL of 1-octanol (Merck) were weighed in a two-necked 100 mL flask under argon atmosphere in dry bag, under constant stirring at 120rpm. Subsequently, the reaction flask, under argon atmosphere, was introduced into an oil bath and heated to 140 to 145°C. These reaction conditions were maintained for 24, 48, 72 and 96hours respectively, while the obtained PLLA acquired a solid consistency.
Purification of high molecular weight PLA
The above process was repeated to obtain 10.0g of PLA, which was dissolved in the least amount of chloroform (Merck), with stirring at room temperature. Then, twice the volume of methanol (Merck) was added to form a milky suspension that, eventually, precipitated. This white solid was filtered in vacuum Buchner funnel to give 9.4g (mp: 92 and 94°C) and finally stored in a dessicator.
Spectroscopic properties and characterization
Thermal analysis: The L-Lc and PLLA were analyzed by differential scanning calorimetry (DSC) and thermogravimetric (TGA) in a TA-modulated SDTQ600 equipment at a heating rate of 10°C /min under nitrogen atmosphere with a flow rate 100mL/min from 35°C to 350°C, using samples weighing approximately 10mg.
Molecular weight determination by capillary viscometry: PLLA molecular weights were determined using a 1C E-999 Ubelholde viscometer (NOOC-200), chloroform (CHCl3) as solvent and solutions with concentrations of PLLA 0.02, 0.01, 0.007, 0.005, 0.003, 0.001g/cm3, in a thermostatic bath at 25°C. Elution times were measured by triplicate for each concentration and the relative viscosity calculated as 1.02793. The specific viscosity was obtained by extrapolating to zero in the plot ηsp/C vs. the square root of the concentration.19 The molecular weight was obtained by the Mark-Houwink-Sakurada equation (1).
Where: κ and α are constants characteristic of each polymer and [η] is the intrinsic viscosity. κ is 6,67x103 and α =0,6720
Electrospinning of PLLA obtained through ROP
The parameters to obtain the fibers were: voltage 20kV, distance 14cm; flow rate of 8mL/min. A 40% solution of PLLA (295,000g/mol) in CHCl3 was prepared and placed in a 5mL syringe attached to a hose, the voltage source (20kV) was connected with a flow rate of 8mL/min. The distance between the needle and the foil was 14cm. The fibers were collected on aluminum boxes 8cm long by 5cm wide and 3cm thick.
Cellular growth
Preparation of PLLA samples for cell growth: The preparation of PLLA samples for cell growth requires most aseptic conditions possible. The laboratory bench was cleaned with 70% ethanol. 45 vials for samples were dried. For the preparation of these scaffolds, a drill was used, so the dimensions of the scaffolds were the same. The scaffolds for cell growth were cut:
These fibrous PLLA scaffolds were used in triplicate for fibroblasts growth, which acts as an extracellular matrix (ECM) where cells may adhere and grow, thus enhancing the development of new tissue, transportation of nutrients and removal of unwanted materials.21
Cytotoxicity test of fibrous scaffolds
An in vitro cytotoxicity test was performed with the LDH Roche®22 kit. This test is a colorimetric test used to quantify cell lysis and it is based on the LDH measure of the activity released from the cytosol of lysed cells in the supernatant. L929 cell line (ATCC CCL1, NCTC clone 929) is a human skin fibroblast line subcloned from the line L.23
Preparation of working solutions
A 1mL of the catalyst aqueous solution was added and stirred for 10minutes. This solution can be kept for weeks at 2-8°C. The dye solution is stored at 2-8°C. For the 100 trials, 100µL of the catalyst with 11.25mL of dye solution was mixed.
Cellular culture
The number of defrosted cells was determined by the tripan blue method and they were seeded in a concentration of 150.000 cells per well in a 24 well (FALCON) plate with a growing medium (Modify medium Eagle´s Dulbeco (DMEM)), supplemented with 10% fetal bovine serum (FBS) (GIBCO BDRL), 2mM L-glutamine buffered saline (PBS). The cell plate was incubated for 48h with CO2 at 37°C, until a growth of 100%.23 After incubation time and further verification of cell confluence, fibrous PLLA scaffolds were added as the final product. All triplicate samples were mounted as shown in Figure 1. The 24-well plate containing the samples described above and cells were incubated at 37°C in CO2 incubator for 24hours.
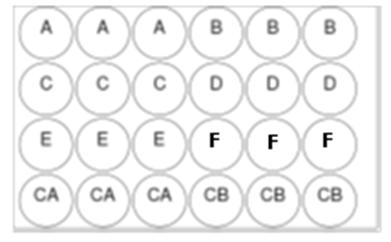
Figure 1 Schematic representation of a 24-well plate. Note that there are three wells on each plate with each of the treatments A, B, C, D, E and F. In addition, arranged in each plate, there were two controls: (1) a high control (CA) and (2) an under control (CB), each in triplicate (three wells).
The arrangement of the materials to be analyzed in 24-well plate was:
A: Cell culture medium (white).
B: Cells + pure PLLA 96 h.
C: Cells + commercial PLLAl.
D: Cells + pure 80 % PLLA 48 h and commercial 20 % PLLA.
E: Cells + pure 90 % PLLA 72 h and commercial 10 % PLLAl.
F: Cells + pure 90 % PLLA 96 h and commercial10 % PLLA.
CA: Cells + culture medium + triton solution.
CB: Cells + culture medium.
Cytotoxicity test with LDH Kit ® ROCHE
After the incubation time, 100µL of supernatant were transferred from each well of the first plate to the corresponding wells in a 96 well plate, 100µL of LDH reagent were added and the incubated plate at room temperature was protected from light for 30minutes. After this time the optical density was determined using a micro-plate reader for ELISA, STAT-FAX 2100, at a wavelength of 456nm with a reference filter 492nm. In order to determine cytotoxicity, the LDH technique was performed with measurements at 72h post incubation with materials and incubation for 30minutes with the LDH reagent with the Stat Fax-2100 at a wavelength of 456nm with a reference wavelength of 490nm.
Commercial LA (88-92%) consists of dimers, trimers and Lc.24 Upon heating a PLA oligomer is formed as shown in Figure 2.
The molecular weight of the formed oligomer can be controlled using different catalysts, functionalizing agents or by changing the polymerization conditions.25 Direct poly-condensation of LA in situ is not applied in an industrial scale because of the competition between the formation of Lc and simultaneous degradation that occurs in the reaction.26,27 The second stage is the de-polymerization of the oligomer to give Lc, as illustrated in Figure 3.
Crude Lc contains impurities such as water, LA, a mixture of oligomers such as L, D and D L Lc and L-Lc, the last of which is in greater proportion. Therefore, it was necessary to recrystallize this mixture. The L-Lc specific rotation (toluene) was measured [α]D=-285.3°C after 10 repetitions, with a standard deviation of 9,366, very close to that reported by McDonald28 and Aldrich:29 [α]D=-285°C.
Characterization of reactants and products
Infrared spectra of materials used in this study are consistent with those reported in the literature and in our own laboratory.30–34
Recrystallization of Lc
The crude Lc and water react together at a relatively low temperature dissolving some impurities such as meso Lc. Additionally, the D and L-Lc are hydrolyzed and separated from de original solution. Thus meso-Lc is easily removed by recrystallization allowing the pure Lc formation, starting material for high molecular weight PLLA.35
Viscometry
The specific viscosity [η] was 30,488 employed in the calculation of the molecular weight using the Mark Houwink Sakurada equation.
(1)
This molecular weight is adequate for electrospinning of the samples.
Thermal analysis of the synthesized compounds
L-lactide: The melting temperature (Tm) was 95.65°C and the decomposition temperature (Td) 195.12°C. The values found in the literature differ greatly for example Fuentes et al.,36 reported a melting temperature of 119.8°C, a difference that could be due to the contribution of DL-racemic mixture. PLLA: The Tg observed was 70.54°C, Tm6°C and the Td is 306.43°C. The thermal characteristics of PLLA are important for manufacturing fibrous scaffolds by electrospinning. A relatively high Tg with respect to the reported 70.54°C shows that the synthesized PLLA is high molecular weight. It should be noted that Tg and Tm increase as the molecular weight increases and are strongly influenced by the degree of crystallinity of PLLA. A relatively high Tg of 68°C for a highly crystalline PLLA was reported by Gupta.26 The Tg for 96 h PLLA obtained by us is very close to this temperature, indicating that the obtained PLLA was highly crystalline. It was also found that the PLLA with a Tg of 70.2°C has a 49% degree of crystallinity, which indicates a satisfactory percentage of crystallinity for our 96 h PLLA, that would make it suitable for electrospinning.37
Thermogravimetric Analysis (TGA) of L-Lc: This analysis was performed on the same machine and the same parameters of the experimental part and results session. It is concluded that L-Lc starts its decomposition at a temperature of 108.5°C with a weight loss of 6.7% and reach a high degree of decomposition at 195, 1°C with a weight loss of 95%.
TGA analysis of PLA 96h: The PLA started decomposition at a temperature of 250.33°C with a rate of 95.03% weight and achieved a high degree of decomposition at 318.3°C with the weight percentage of 1.81%.
Electrospinning
The parameters used are similar to those reported by Zong et al.38 A 40% (W/V) of a 90:10 mixture of PLA molecular weight 295,000g/mol and commercial PLA was prepared and subjected to electrospinning and the fibers were collected on aluminum rectangular boxes. These samples were used for cell growth.39
Cytotoxicity tests
To calculate cytotoxicity is necessary to check the three controls in this experiment: the first control is the cell culture medium, which provides information on the activity of LDH. The absorbance value obtained in this control should be subtracted from the values of the other controls. The second control is labeled as “under control”, which provides information on the activity released from untreated normal cells. The third control is labeled as the “high control”, which provides information on the maximum activity of the LDH enzyme released.
Finally the equation (2) is used to determine the percentage of cytotoxicity of each of the compounds tested.
(2)
The percentage cytotoxicity is calculated by applying equation (2) to all samples. Table 1 shows the results of cytotoxicity of PLLA.
Muestras |
Cytotoxicity (%) |
PLLA 96 h |
49, 34±0.01 |
PLLA comercial |
23,00±0.01 |
80 % PLLA 48 h pure y 20 % PLLA commercial |
42,76±0.01 |
90 % PLLA 72 h pure y 10 % PLLA commercial |
32,12±0.01 |
90 % PLLA 96 h pure y 10 % PLLA commercial |
42,93±0.01 |
Table 1 Results of the cytotoxicity test of the PLLA samples
According to Table 1, the synthesized PLLA have the greatest value of all. The commercial PLLA has the lowest value. Nevertheless, Lozano (2013)40 argues that the fibrous blocks made of PLLA by less than 50% are not considered cytotoxic. Therefore, all PLLA synthesized in this study did not exhibit cytotoxicity and are considered useful in applications with living cells. The next step is to carry our histological tests in vivo.
We found that mixtures of synthesized PLA (295,000g/mol) with commercial PLA, 90/10 by weight were successfully processed by electrospinning obtaining fibrous matrices that gave favorable results in cytotoxicity tests with human skin fibroblasts.
The authors thank the Center of Excellence New Materials (CENM) and the Universidad Del Valle for financial support to successfully complete this investigation.
The author declares no conflict of interest.

©2016 Peñaranda-Armbrecht, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.