MOJ
eISSN: 2374-6939


Case Report Volume 10 Issue 1
1Department of Orthopaedics, King Fahad Hospital in Hofuf, Kingdom of Saudi Arabia
2Department of Orthopaedic Surgery, King Hamad University Hospital, Kingdom of Bahrain
3College of Medicine, King Faisal University, Kingdom of Saudi Arabia
Correspondence: Eyad Alqasim, King Hamad University Hospital, P.O. Box 24343, Muharraq, Bahrain, Tel 97333366903
Received: January 25, 2018 | Published: February 5, 2018
Citation: Alsaleem MK, Alqasim E, Aleid ZM, Alhathloul HS, Alharbi NM et al. (2018) A Unique Adult-Type Distal Tibia Non-Ossifying Fibroma with Secondary Aneurysmal Bone Cysts Presenting with a Pathological Fracture. MOJ Orthop Rheumatol 10(1): 00387. DOI: 10.15406/mojor.2018.10.00387
Non-ossifying fibroma is a benign tumor primarily of childhood and adolescence. It is the most common tumor encountered in the pediatric population. A high index of suspicion should always be maintained in order to make the correct diagnosis. Although the expected adulthood hypersclerosis usually takes place in the primary lesion, its absence should never eliminate this entity from the differential diagnosis owing to its characteristic radiological appearance and natural history. The occurrence of such entity in an adult with already closed physis; and in less common locations; e.g. distal tibia, raises the challenge for appropriate interpretation of the radiological features. A better understanding of the pathophysiology of the disease will certainly enhance our approach. We present a unique case of an adult-type non-ossifying fibroma with secondary aneurysmal bone cyst changes of the distal tibia that was complicated by a pathologic fracture. Although described in literature, to our knowledge and following extensive research, it is rarely well-documented.
Keywords: Benign tumor, Non-ossifying fibroma, Pathological fracture, Aneurysmal bone cyst, Fibrous cortical defect, Cortical desmoid
Various terms have been suggested to describe non-ossifying fibroma including fibrous cortical defect, cortical desmoid, metaphyseal fibrous defect and non-osteogenic fibroma. Regardless of the term used, they probable all refer to abnormal osteoclastogenesis at the metaphyseal region. Non-ossifying fibroma is a benign fibrogenic tumor primarily affecting children and adolescents. It is the most common childhood benign tumour in which it is found in 30% of children with open physis. It initially affects the metaphysis of long bones, most commonly the distal femur followed by the proximal and distal tibia. However, as growth takes place, the lesion extends into the diaphysis and once close to skeletal maturity it transforms into a hypersclerotic scar.
Clinical features are usually unremarkable and the patient is asymptomatic, unless the lesion is complicated by a fracture. The lesion may easily be diagnosed by plain film radiographs due to its characteristic metaphyseal lytic lobulated pattern that is surrounded by a well-defined sclerotic rim. Although not required for the diagnosis, histopathological examination may demonstrate characteristic spindle cells with elongated nuclei in a fibroblastic connective tissue matrix with abundant lipophages giant cells and hemosiderin pigmentation.
The mainstay of treatment is conservative. In secondary pathological fractures, however, the approach depends on patient age. In very young patients, a simple full cast may suffice to stabilize the fracture, while in older children curettage and surgical fixation may be necessary.
A 23-year-old healthy male presented to the accident & emergency department complaining of pain in the right lower leg and ankle following a trivial twisting injury to the right ankle while training in the gym. On examination, the patient had severe pain and mild swelling to the right lower leg and ankle with no visible deformity, normal skin condition and intact distal neurovascular status. Initial radiologic examination revealed a distal third tibia and fibula long oblique displaced fractures that were associated with a roughly 3cm x 2cm well-defined exapnsile, lobulated and lytic lesion with hypersclerotic margins and a narrow transitional zone (Figure 1). Routine laboratory investigations including a complete blood count, liver function test and renal function test did not reveal any abnormalities. An initial diagnosis of pathological fracture with a differential diagnosis of non-ossifying fibroma versus chondromyxoid fibroma was, therefore, made. The patient was, accordingly, planned for bone curettage and primary fixation of the distal tibia and fibula.

Figure 1 Initial injury AP and lateral x-rays - distal tibia and fibula fractures with an associated expansile, lobulated and lytic lesion with hypersclerotic borders.
The fracture was first approached via an anterolateral approach to the distal fibula in which the fibula fracture was fixed by a 1/3 tubular plate and screws. This was followed by a complete curettage of the lesion and bone grafting with cancellous bone chips allograft. Finally, the tibia fracture was fixed by minimally invasive percutaneous osteosynthesis (MIPO) technique using 3.5mm locked plate medially (Figure 2). The excised bone material was then sent for histopathological examination which confirmed a non-ossifying fibroma with secondary aneurysmal bone cysts (Figures 3- 5).
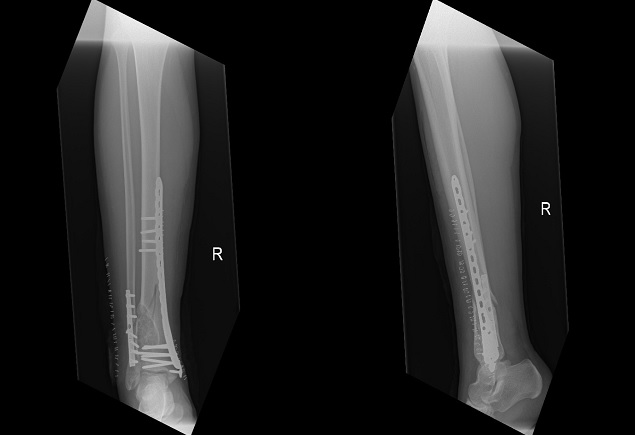
Figure 2 Immediate post-operative AP and lateral x-rays - fibula fracture fixed with a 1/3 tubular plate and screws with the lesion curretaged and filled with allograft; and the tibia fixed by MIPO plate and screws.
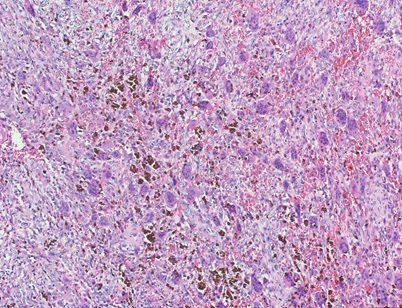
Figure 3 Histopathological slide of the lesion-high-power magnification revealing abundant osteophytes and hemosiderin pigmentation.
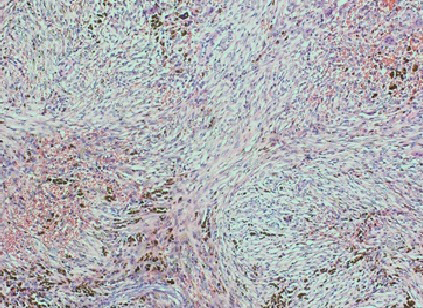
Figure 4 Histopathological slide of the lesion-high-power magnification showing characteristic spindle cell fibroblastic proliferation consistent with non-ossifying fibroma.
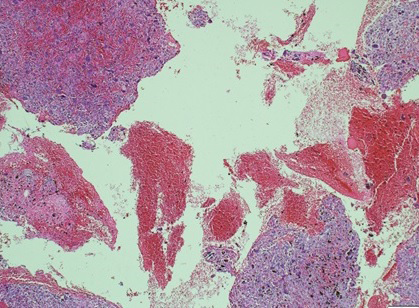
Figure 5 Histopathological slide of the lesion-high-power magnification demonstrating blood-filled spaces consistent with secondary aneurysmal bone cysts.
The patient was initially placed on a non-weight bearing protocol for eight weeks with only crutch and touch-toe ambulation. Follow-ups were carried out at two weeks, four weeks, eight weeks, sixteen weeks, eight months and one year post-operatively. At eight weeks, the ankle range of motion was regained and a healing callous started to form. The patient was, therefore, allowed to partially bear weight. At 4 months post-operatively, full weight bearing was started when the patient had a painless full range of ankle motion and x-rays revealed good incorporation of the allograft (Figure 6). Examination at eight months and 1 year post-operatively revealed full ankle strength and range of motion restoration with a complete filling of the defect and total fracture healing (Figure 7).
It is well recognized that non-ossifying fibroma is a benign tumor occurring mainly in childhood and adolescence.1 Although uncommon, we agree with Glockenberg et al. who described in their paper that the age range for this condition is 3 to 42.1 Most literature state that there is a higher male predominance to females; and this was estimated to be 2:1.1,2 The condition most commonly affects the metaphyseal region of bone,1 namely the distal femur followed by the proximal tibia.1,3
Ritschl et al.3 carried out extensive studies to determine the site of evolution of non-ossifying fibroma.3 Accordingly, it was concluded that non-ossifying fibroma probably arises from the insertion sites of tendons or ligaments in the metaphysis.3 Ritschl et al.3 have also developed a radiological classification system for non-ossifying fibroma which adequately demonstrates how the lesion transforms from its primitive lobulated and lytic pattern to its well healed sclerotic form. According to this classification, stage A includes small, oval, possible slightly lobulated lesions without sclerotic margins. These are usually eccentrically located in the cortex near the physis. Stage B includes lesions that are both lobulated and sclerotic. The location of these lesions varies depending on the rate of growth. In stage C, the lesion becomes mineralized. Finally, stage D includes fully sclerotic lesions. This classification system indeed proves the rarity of occurrence of the classical early and primitive features of the lesion in adulthood as was demonstrated in our patient.
The lesion is usually asymptomatic, but it is not uncommon to encounter non-ossifying fibroma patients presenting with at least minimal symptoms such as mild discomfort, especially if the lesion is large or associated with some degree of trauma.1,4 The presence of pain may be related to compression to the surrounding tissue and/or weakening of the bone.5
The authors agree with Arata et al.6 who suggested that the risk of developing a pathological fracture in a non-ossifying fibroma bone increases if the primary lesion is more than 33mm long or occupies more than 50% of the transverse diameter of the bone.6 This conclusion may be applied adequately to our patient whose initial radiographs perfectly demonstrated such findings.
The diagnosis is usually incidental during radiological evaluation for other reasons.4 Due to its characteristic radiological appearance, pathological confirmation is usually not necessary.5,7 This is especially true when the lesion demonstrates characteristic features such as a longitudinal growth pattern, lobulated and bubbly appearance with sclerotic border, and intact cortex.7 If however, any of these features is breached or in complicated cases, biopsy may be indicated.7 Histologically, the lesion reveals storiform fibrous tissue with spindle cells, multinucleated giant cells, hemosiderin and xanthoma cells.8 Secondary aneurysmal bone cysts are usually not present in the primary lesion. The presence of such changes is probably attributed to the complicated pathological fracture which results in bleeding and the formation of blood-filled spaces.7
We strongly agree with Andreacchio et al.5 in prescribing surgery for symptomatic cases or cases complicated by a pathological fracture.5 We believe that Arata’s criteria are a very useful tool that when combined with the clinical presentation can provide reliable guidelines to appropriately determine the necessity of surgical intervention. These guidelines have, therefore, been implemented in our patient who accordingly constituted an excellent surgical candidate. Some controversy, however, still exists on the need for surgical treatment in pathological fractures. Drennan et al.9 recommended curettage and bone grafting because the primary lesion would persist and not be obliterated as the fracture heals.9 Gitelis et al. on the other hand, stated that lesion regression occurs as the fracture heals.10 The authors, however, believe that curettage, bone grafting and fracture fixation is inevitable. The choice of bone grafting is various with calcium sulphate,5 autogenous bone marrow grafts11 and, in our case, cancellous bone chips being used.
None.
The authors declare that they have no competing interests.
The patient presented in this case report has given his/her full informed consent for the case report to be published.

©2018 Alsaleem, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.