MOJ
eISSN: 2374-6939


Review Article Volume 1 Issue 3
1Regeneration Technologies, USA
2University of South Carolina, Greenville School of Medicine, USA
Correspondence: Henry E Young, Regeneration Technologies LLC, 778 Mulberry Street, Macon, GA 31201, USA, Tel 4783191983
Received: October 28, 2014 | Published: November 26, 2014
Citation: Young HE, Black AC. Pluripotent stem cells, endogenous versus reprogrammed, a review. MOJ Orthop Rheumatol. 2014;1(3):72-90. DOI: 10.15406/mojor.2014.01.00019
There are two general categories of Pluripotent stem cells, endogenous Pluripotent stem cells and reprogrammed Pluripotent stem cells. Endogenous Pluripotent stem cells are formed during development. There are two subcategories of endogenous Pluripotent stem cells, embryonic stem cells (ESCs) and postnatal (“adult”) stem cells (ASCs). Reprogrammed Pluripotent stem cells are derived by either somatic cell nuclear transfer (SCNT) whereby there is the transfer of the nucleus from a differentiated cell into the cytoplasm of an enucleated oocyte or by the insertion of specific genes into terminally differentiated cells to artificially induce them to express attributes of more primitive pluripotent stem cells (induced Pluripotent stem cells, iPSCs). This review outlines the developmental process and differentiative capabilities of endogenous pluripotent stem cells; the manufacture and differentiative capabilities of reprogrammed Pluripotent stem cells; and the inherent characteristics of endogenous and reprogrammed Pluripotent stem cells.
Keywords: Pluripotent stem cells, Somatic cells, Transplantation
ESC, Embryonic Stem Cells; ASC, Adult Stem Cells; SCNT, Somatic Cell Nuclear Transfer; IPSC, Induced Pluripotent Stem Cells; APUD, Amine Precursor Uptake Decarboxylase; LIF, Leukemia Inhibitory Factor; GFP, Green Fluorescent Protein; VCAM, Vascular Cell Adhesion Molecule; PECAM1, Platelet Cell Adhesion Molecule 1; MAPCS, Multipotent Adult Progenitor Cells; VSELs, Very Small Embryonic-Like Stem Cells; SSEA, Stage Specific Embryonic Antigen; CEA-CAM-1, Carcinoembryonic Antigen-Cell Adhesion Molecule-1; MIAMI, Marrow-Isolated Adult Multilineage Inducible; HBMSCs, Human Bone Marrow-Derived Multipotent Stem Cells; FSSCs, Fetal Somatic Stem Cells; SCNT, Somatic Cell Nuclear Transfer; ICSI, Intra-Cytoplasmic Sperm Injection; BLSCs, Blastomere-Like Stem Cells
To understand the inherent differentiative plasticity of pluripotent stem cells, one needs to understand the normal developmental process that creates an individual from the fusion of two haploid gametes to the formation of the more than 220 differentiated parenchymal cells (functionally active cells), stromal cells (that form the supportive connective tissue framework), and their associated precursor cell types of the body. The normal developmental process from embryonic cell stages to a fully differentiated individual progresses through a defined sequence of differentiation events (Figure 1).1
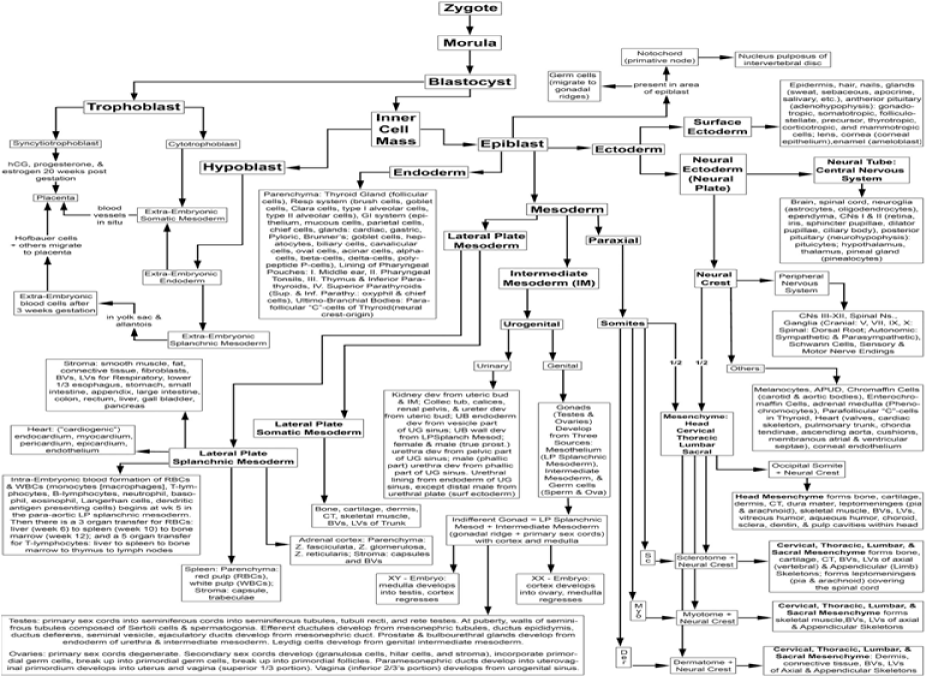
Development in mammals involves syngamy (fusion) of two haploid gametes to form the zygote, a fused diploid totipotent cell.2,3 The totipotent zygote is a self-contained entity that can give rise to an entire organism capable of procreating its species. It has the potential to form a three-dimensional embryo, as well as the extra-embryonic supportive placental tissues necessary for fetal development and the haploid gametes. This inherent ability to form an entire organism complete with placental membranes and gametes is present at least through the 4-cell stage embryo4 and has been demonstrated experimentally in mice, rats, cows and rhesus monkeys5–8 after transfer of single blastomere from 4-cell stage embryos into a suitable host. Blastomeres appear to lose the capability to form an entire organism as development progresses to the 8-cell stage and beyond.3,4
Subsequent mitotic division from the 4-cell stage results in the formation of the morula, a solid ball of blastomeres (Figure 2). Further development leads to the formation of the blastocyst, a hollow sphere. The blastocyst differentiates into an inner cell mass, trophoblast, and gametes. The trophoblast will segregate into cytotrophoblast and syncytiotrophoblast and eventually form the extra embryonic placental tissues. The inner cell mass will segregate into the hypoblast and the pluripotent epiblast. The hypoblast will form the other portion of the extra embryonic tissues/cells (extra embryonic endoderm, extra embryonic splanchnic mesoderm and Hofbauer cells).3 The pluripotent epiblast, with the plasticity to form all somatic cells of the body, will segregate via gastrulation into the ectodermal, mesodermal and endodermal germ cell lineages (Figure 1).1,3 The ectoderm will further differentiate into surface ectoderm and neural ectoderm. The surface ectoderm will form the epidermis, hair, nails, enamel of the teeth, lining of the mouth, exocrine glands, adenohypophysis, lens, cornea, etc. The neural ectoderm will form the neural tube and neural crest. The neural tube will form all the tissues and cells of the central nervous system (brain and spinal cord), including neurons, their supportive cells (the oligodendrocytes and astrocytes), ependymal, olfactory nerve, optic nerve, retina, iris, pineal, epithalamus, thalamus, hypothalamus, neurohypophysis, etc. The neural crest will form tissues/cells of the peripheral nervous system (cranial nerves III-XII, dorsal root ganglia, autonomic ganglia, Schwann cells, sensory and motor nerve endings), as well as melanocytes, amine precursor uptake decarboxylase (APUD) cells, chromaffin cells, enterochromaffin cells, adrenal medulla, parafollicular “C” cells, corneal endothelium and associated structures of the heart and great vessels (cardiac skeleton, atrial septae, ventricular membranous septum, cardiac cushions, heart valves, chordae tendineae, pulmonary trunk, ascending aorta), corneal endothelium (Figure 1).1,3

The mesoderm segregates into paraxial mesoderm, forming the somites; intermediate mesoderm, forming the urogenital systems; and lateral plate mesoderm, forming the stroma of the viscera, lateral body wall, and appendages. Collectively, the mesodermal derivatives form the dermis, muscle (skeletal, cardiac, smooth), bone (cortical, trabecular), cartilage (hyaline, articular, growth plate, elastic, fibrocartilage), fat (unilocular adipose tissue, multilocular adipose tissue), connective tissues (loose fibrous connective tissue comprising the serosa, adventitiaand areolar connective tissues; dense fibrous connective tissue comprising organ capsules, trabeculae, aponeuroses, tendons, ligaments), hematopoietic cells, heart (inflow tracts, endocardium, myocardium, epicardium, Purkinje fibers, conduction system, pericardial sac), blood vessels, lymphatic vessels, spleen, kidneys, ureters, urinary bladder, urethra in females and prostatic urethra, membranous urethra and spongy urethra in males; and stroma for the female and male reproductive organs including the gonads, respiratory system, and gastrointestinal system (lower third of the esophagus, stomach, small intestine, large intestine, cecum, appendix, liver, gall bladder, and pancreas. With respect to the axial skeleton (head and vertebral column) the occipital, cervical, thoracic, lumbar and sacral somites join with neural crest to form vitreous humor, aqueous humor, choroid, sclera, and dentin and pulp cavities of the teeth, dura mater, arachnoid mater, pia mater, etc. (Figure 1).1,3 The endoderm will segregate into the lining of the pharyngeal pouches, forming the middle ear, pharyngeal tonsils, parathyroid glands, thymus gland and parenchyma of the thyroid gland, respiratory system, pancreas, liver, gall bladder, and digestive tract, (i.e., stomach, small intestine, appendix, large intestine, cecum, appendix, and rectum) (Figure 1).1,3
Precursor cells
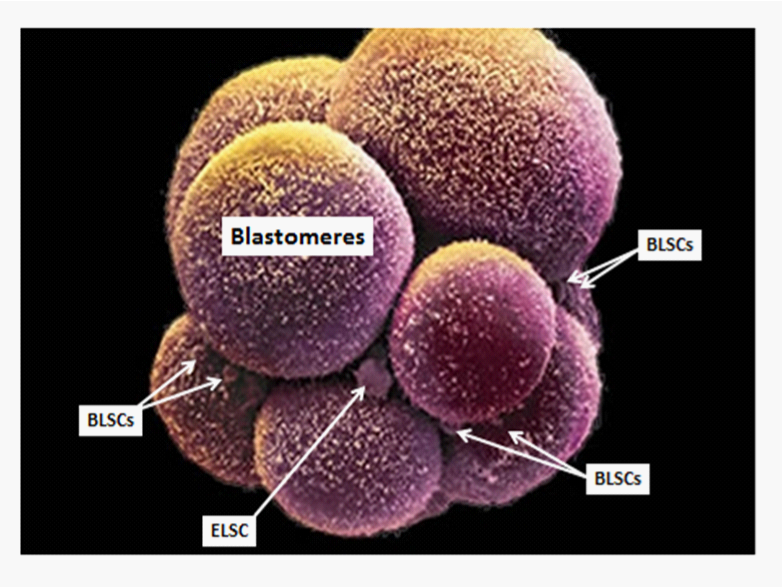
Remarkably, while the vast majority of developing blastomeres transition through the sequence of developmental and differentiation events depicted above for embryogenesis, a few cells become reserve precursor cells (Figure 3)9 that provide for continual maintenance (progenitor cells)and repair (stem cells)of the organism. The totipotent and pluripotent stem cells can be seen as early as the morula stage in human embryonic development (Figure 4). Totipotent stem cells are equivalent in differentiation potential to the blastomeres of the morula and blastocyst;9 pluripotent stem cells are equivalent in differentiation potential to the cells of the epiblast;10 and the germ layer lineage stem cells (ectodermal stem cells, mesodermal stem cells and endodermal stem cells) are equivalent in differentiation potential to the cells comprising the ectoderm, mesoderm, and endoderm germ layer lineages, respectively.11

Assisted Reproductive Technologies (ARTs)
Assisted reproductive technologies were developed to assist infertile couples in the creation of their own biological children. The first successful report occurred after the birth of Mary Louise Brown in 1978. Assisted reproductive technologies span a range of techniques from placement of sperm into the fallopian tube to fertilize the ovulated ovum, termed gamete intra-fallopian (intra-tubal) transfer, to more advanced techniques, such as ex vivo intra-cytoplasmic sperm injection (ICSI) of the haploid sperm nucleus into a haploid ovum to form a diploid zygote, with its subsequent growth in vitro to create a morula before transplantation. Utilizing ARTs procedures in which the initial stages of embryogenesis occur in the culture dish, a blastomere from each diploid morula is tested genetically to discern any genetic mutations or inborn errors of metabolism that would engender congenital malformations in the resultant individual. Blastocysts that are free of any genetic defects are then placed into the uterus of the biological mother or a surrogate host to implant within the endometrium and further its development. This technology creates biological children with a genetic makeup similar to that of their respective donors of sperm and ova. The blastocysts that are formed during these processes have an inherent preprogramming within their genome for development and spatial arrangement to collectively create an individual composed of multiple differentiated cell types for the overall functioning of the individual in time and space and endogenous precursor cells for the maintenance and repair of the individual’s body tissues.3,12–16
Embryonic Stem Cells (ESCs)
Populations of embryonic stem cells (ESCs) were initially created to have sufficient cells of the same genetic makeup to study cellular aspects of embryogenesis. Evans and Kaufman17 and Martin18 were the first to report the derivation of embryonic stem cells from murine embryos. The creation of knock-out mice for the analysis of mammalian gene function analysis was made possible by these discoveries. Their technology entailed harvesting blastocysts from pregnant breeders, separating the inner cell mass from the trophoblast and dispersing the inner cell mass cells. The dispersed cells were then plated into culture dishes in the presence of a feeder layer or of an inhibitor such as leukemia inhibitory factor (LIF) or ESGRO to prevent spontaneous differentiation of the blastomeres. The differentiated-inhibited blastomeres were then stimulated to proliferate to expand their numbers. During this expansion process collections of cells occurred, termed embryoid bodies. When sufficient numbers of embryoid bodies were formed, the inhibiting agent was removed and the embryoid bodies underwent uncontrolled spontaneous differentiation to form masses of cell types from all three germ layer linages, (i.e., ectoderm, mesoderm and endoderm). The cell types formed within the ectodermal layer included neurons, neuroglia, epidermis, hair, teeth, and nails. Mesodermal tissues formed were muscle, fat, cartilage bone, connective tissues, endothelial cells and blood cells. Endodermal lineage tissues included gastrointestinal epithelium, hepatocytes, and pancreatic cells (α-cells, β-cells, δ-cells, and ductal cells).19 Thus, these embryonic stem cells were classified as pluripotent stem cells for their ability to form cells from all three primary germ layer lineages as well as having the capacity for unlimited proliferation ex vivo.20 When ESCs were placed into an animal they would form a mass of tissue that contained a variety of cell types in a non-spatially arranged jumbled fashion. This configuration is reminiscent of the sacrococcygeal teratoma that can form during embryogenesis if residual primitive streak cells remain after gastrulation at the caudal end of the developing embryo, located caudal to the notochord (the primary inducer of the embryo).3 Therefore, the in vivo placement of ESCs within an individual with the resultant uncontrolled spontaneous differentiation of multiple cell types was termed a teratoma and forms the hallmark verification protocol for ESCs. By 2009, over 10,000 mutated mice were generated world-wide using these gene targeting techniques.4 The reports of mouse embryonic stem cells spurred the development of embryonic stem cells in other species. Thomson et al. 21 reported the successful derivation of embryonic stem cells from blastocysts of the rhesus macaque. The cells displayed pluripotency in their ability to form cell types from all three primary germ layer lineages: ectoderm, mesoderm and endoderm. Other investigators reported the derivation of embryonic stem cells from non-human primates, i.e., marmosets,22 cynomolgus macaques,23 and an additional 25 cell lines in the rhesus macaque.24
The derivation of ESCs utilizing similar technologies have been verified by other labs in mice and generated from blastocysts of rat, golden hamster, rabbit, mink, pig, sheep, cow, horse, marmoset, non-human primate (Rhesus monkey), human, chicken, medaka, zebra fish and gilthead sea bream.16 Utilizing protocols and markers previously developed in non-human primates,22 Thomson et al.25 and shamblott26 reported the isolation and characterization of embryonic stem cell lines derived from surplus human embryos created by ARTs. Shamblott and Gearhart26 reported the generation of ESCs from primordial germ cells from the genital ridge of aborted human fetuses. In both instances, the created ESCs proved to be pluripotent in their ability to form differentiated cell types from all three primary germ layer lineages, although neither group reported the formation of gametes from their ESCs. The lack of gamete production suggested that the particular cell types used were a more downstream cell type from the more upstream undifferentiated totipotent morula blastomeres. Subsequent studies in mouse, pig, cow, human and chicken have shown gamete production from morula-derived ESCs.27
Since the initial report of Thomson et al.25 Shamblott et al.,26 multiple laboratories have been attempting to direct differentiation of the embryoid bodies derived from ESCs into selected cell types for tissue repair and replacement. One of the more exquisite experiments reported27 generation of pancreatic β-cells from ESCs for the treatment of insulin-dependent diabetes. In this experiment ESCs were transfected with a green fluorescent protein (GFP) reporter sequence downstream of the gene sequence for the formation of pancreatic β-cells. After proliferation of the transfected ESCs in the presence of an inhibitor with embryoid body formation, the inhibitor was removed and the resultant embryoid bodies were allowed to spontaneously differentiate. The differentiated cell types were dispersed and only those cells that fluoresced green (β-cells only) were collected and utilized for insulin synthesis and secretion after a sequential glucose challenge for proof of principal according to Lumelsky et al.28 Parallel studies, inducing embryonic stem cells to form insulin-secreting beta cells have been reported and refined by Melton et al.29,30
Adult precursor cells
Locations
Due to their appearance early in development and segregation with the developing blastomeres, precursor cells with similar differentiation potentials as those described for embryonic cells have been postulated to occur in most organs and tissues of the body throughout the lifespan of the individual. Indeed, studies encompassing isolation from solid tissues and blood as well as cryosectioning and immunocytochemical staining of tissues with antibodies specific for precursor cell-specific antigen markers (Table 1), demonstrated that reserve precursor cells are present in postnatal mammals such as the mouse, rat, rabbit, cat, dog, goat, sheep, pig, cow, horse, and human (Table 2).31 These precursor cells were composed of both progenitor cells and stem cells. The stem cells included totipotent stem cells, Pluripotent stem cells, and the germ layer lineage ectodermal stem cells, mesodermal stem cells, and endodermal stem cells (Table 3).9–11,31–33
|
Antibodies used for Characterization of Cell Types9,10,11,31-33,46,94 |
||
|---|---|---|
|
Antibody |
Antigen |
Embryological Origin |
|
CEA-CAM-1 |
Carcinoembryonic antigen-cell adhesion molecule-1 |
Totipotent |
|
HCEA |
Human Carcinoembryonic antigen |
Totipotent |
|
CEA |
Carcinoembryonic antigen |
Totipotent |
|
CD66e |
Carcinoembryonic antigen |
Totipotent |
|
DH-TuAg1 |
Spermatogonia |
Totipotent |
|
MC-480 |
SSEA-1 |
Pluripotent |
|
MC-631 |
SSEA-3 |
Pluripotent |
|
MC-813 |
SSEA-4 |
Pluripotent |
|
CD10 |
Neutral endopeptidase |
Pluripotent |
|
AlkPhos |
Alkaline Phosphatase |
Pluripotent |
|
CD90 |
Thy-1 |
Germ Layer Lineage |
|
CD56 |
Neural cell adhesion molecule |
Ectoderm |
|
Pax-6 |
Neurogenic lineage |
Ectoderm |
|
FORSE-1 |
Neuronal precursor cells |
Ectoderm |
|
Vimentin |
Cells of neurogenic lineage |
Ectoderm |
|
Nestin |
Cells of neurogenic lineage |
Ectoderm |
|
R401 |
Nestin-neuronal lineage |
Ectoderm |
|
HNES |
Nestin-neuronal lineage |
Ectoderm |
|
MAB353 |
Nestin-neuronal lineage |
Ectoderm |
|
RT-97 |
Neurofilaments = neurons |
Ectoderm |
|
NF68 |
Neurofilament-68 = neurons |
Ectoderm |
|
S-100 |
Neurofilament-100 = neurons |
Ectoderm |
|
NF145 |
Neurofilament-145 = neurons |
Ectoderm |
|
N-200 |
Neurofilament-200 = neurons |
Ectoderm |
|
8A2 |
Neurons |
Ectoderm |
|
NG2 |
Neurons |
Ectoderm |
|
TH |
Tyrosine hydroxylase, precursor to neural transmit |
Ectoderm |
|
SV2 |
Synaptic vesicles |
Ectoderm |
|
DOPA |
Dopamine, transmitter of dopaminergic neurons |
Ectoderm |
|
T8660 |
Beta-tubulin-III |
Ectoderm |
|
Tuj1 |
Beta-tubulin-III |
Ectoderm |
|
GFAP |
Glial-fibrillary acidic protein |
Ectoderm |
|
CNPase |
Glial cells = oligodendrocytes & astrocytes |
Ectoderm |
|
Rip |
Oligodendrocytes |
Ectoderm |
|
MOSP |
Oligodendrocytes specific protein |
Ectoderm |
|
MAB |
Oligodendrocytes marker |
Ectoderm |
|
40E-C |
Radial cells and radial glial cells |
Ectoderm |
|
VM-1 |
Keratinocytes |
Ectoderm |
|
CD13 |
Amino endopeptidase |
Mesoderm |
|
OP-137 |
MyoD |
Mesoderm |
|
F5D |
Myogenin = skeletal muscle |
Mesoderm |
|
MF-20 |
Sarcomeric myosin = skeletal muscle |
Mesoderm |
|
MY-32 |
Skeletal muscle fast myosin = skeletal muscle |
Mesoderm |
|
ALD58 |
Myosin heavy chain |
Mesoderm |
|
A4.74 |
Myosin fast chain |
Mesoderm |
|
IA4 |
Smooth muscle alpha actin = smooth muscle |
Mesoderm |
|
Calp |
Calponin |
Mesoderm |
|
MAB-3252 |
Cardiotin = cardiac myocytes |
Mesoderm |
|
MAB1548 |
Myosin heavy chain of cardiac muscle |
Mesoderm |
|
WV1D1 |
Bone sialoprotein II = bone |
Mesoderm |
|
MP111 |
Osteopontine = bone |
Mesoderm |
|
Von Kossa |
Stains calcium in bone |
Mesoderm |
|
CIIC1 |
Type-II collagen = cartilage |
Mesoderm |
|
II-4CII |
Type-II collagen = cartilage |
Mesoderm |
|
HC-II |
Human type-II collagen = cartilage |
Mesoderm |
|
Alcian Blue |
Stains anions on carbohydrate groups |
Mesoderm |
|
AB 1.0 |
Alcian Blue, pH 1.0 stains sulfate groups on GAGs |
Mesoderm |
|
AB 2.5 |
Alcian Blue, pH 2.5 stains carboxyl groups on GAGs |
Mesoderm |
|
Alcec Blue |
Stains anions on carbohydrate groups |
Mesoderm |
|
AcB 1.0 |
Alcec Blue, pH 1.0 stains sulfate groups on GAGs |
Mesoderm |
|
AcB 2.5 |
Alcec Blue, pH 2.5 stains carboxyl groups on GAGs |
Mesoderm |
|
Safranin-O |
Stains anions on carbohydrate groups |
Mesoderm |
|
SO 1.0 |
Safranin-O, pH 1.0 stains sulfate groups on GAGs |
Mesoderm |
|
SO 2.5 |
Safranin-O, pH 2.5 stains carboxyl groups on GAGs |
Mesoderm |
|
HC-II |
Human collagen type-II stains cartilage |
Mesoderm |
|
D1-9 |
Type-IX collagen = cartilage |
Mesoderm |
|
9/30 |
Cartilage link protein |
Mesoderm |
|
12/21 |
Cartilage proteoglycan-hyaluronate binding region |
Mesoderm |
|
12C5 |
Versican hyaluronate binding region |
Mesoderm |
|
H-CD34 |
Sialomucin-containing hemato/endothelial cells |
Mesoderm |
|
CD31 |
PECAM, Peripheral endothelial cell adhesion molecule |
Mesoderm |
|
P1H12 |
Human endothelial cell surface marker |
Mesoderm |
|
P2B1 |
Peripheral endothelial cell adhesion molecule |
Mesoderm |
|
P8B1 |
VCAM, Vascular cell adhesion molecule |
Mesoderm |
|
P2H3 |
CD62e, E-selectin (vasculature) |
Mesoderm |
|
H-endo |
CD146, endothelial cells |
Mesoderm |
|
H5A4 |
CD11b, granulocytes, monocytes, NK-cells |
Mesoderm |
|
H4C4 |
CD44, hyaluronate receptor |
Mesoderm |
|
Hermes-1 |
CD44, hyaluronate receptor |
Mesoderm |
|
H5A5 |
CD45, all hematopoietic cells except RBCs |
Mesoderm |
|
H5C6 |
CD63, macrophages, monocytes, platelets |
Mesoderm |
|
HFSP |
Human fibroblast specific protein |
Mesoderm |
|
1B10 |
Fibroblast-specific protein |
Mesoderm |
|
Sudan Black-B |
Stains fat (adipocytes) |
Mesoderm |
|
Oil Red-O |
Stains fat (adipocytes) |
Mesoderm |
|
H-AFP |
Human alpha-fetoprotein = fetal liver |
Endoderm |
|
R-AFP |
Rat alpha-fetoprotein = fetal liver |
Endoderm |
|
DESMO |
Endodermal epithelial marker of liver |
Endoderm |
|
LAP |
Canalicular cell surface protein of liver |
Endoderm |
|
151-Ig |
Liver epithelial growth factor |
Endoderm |
|
HA4c19 |
Bile canalicular cells of liver |
Endoderm |
|
OC2 |
Progenitor cells, oval cells & biliary cells of liver |
Endoderm |
|
OC3 |
Progenitor cells & biliary cells of liver |
Endoderm |
|
OC4 |
Progenitor cells & biliary cells of liver |
Endoderm |
|
OC5 |
Progenitor cells & biliary cells of liver |
Endoderm |
|
OC10 |
Progenitor cells & biliary cells of liver |
Endoderm |
|
H.4 |
Intracellular staining of liver hepatocytes |
Endoderm |
|
H.1 |
Liver hepatocytes cell surface marker |
Endoderm |
|
DPPIV |
Progenitor, canalicular and biliary cells of liver |
Endoderm |
|
OV6 |
Biliary and oval cells of liver, hepatocyte canalicular cells |
Endoderm |
|
HESA |
Human GI (Gastrointestinal) Epithelium |
Endoderm |
|
cells |
Glucagon-secreting cells of endocrine pancreas |
Endoderm |
|
YM-PS087 |
Glucagon-secreting cells of endocrine pancreas |
Endoderm |
|
cells |
Insulin- secreting cells of endocrine pancreas |
Endoderm |
|
YM-PS5088 |
Insulin- secreting cells of endocrine pancreas |
Endoderm |
|
cells |
Somatostatin-secreting cells of endocrine pancreas |
Endoderm |
|
11180 |
Somatostatin-secreting cells of endocrine pancreas |
Endoderm |
|
CK-19 |
Ductal cells of endocrine pancreas |
Endoderm |
|
PI |
Propidium iodide |
Nucleated Cells |
|
DAPI |
|
Nucleated Cells |
|
Gal-19 |
Insect beta-galactosidase, genomic marker |
Labeled Cells |
Table 1 Antibodies used for Characterization of Cell Types
Char, Characteristics Tested; BLSCs, Blastomere Like Stem Cells; Tr-BLSC/ELSCs, Transitional Blastomere-Like Stem Cell/Epiblast Like Stem Cells; ELSCs, Epiblast Like Stem Cells; Tr-GLSCs, Transitional Germ Layer Lineage Stem Cells; MesoSCs, Mesodermal Germ Layer Lineage Stem Cells; Mes PCs, Mesenchymal Progenitor Cells; Pos, Positive; Neg, Negative; Days Viab PM, Viability Post Mortem; Viab T, Viability Temperature; Tiss, Stem Cells Within Tissues; Skeletal Muscle (Epimysium, Perimysium, Endomysium), Dermis, Heart (Epicardium, Myocardium, Endocardium), Nerve Sheaths (Epineurium, Perineurium, Endoneurium), Granulation Tissues, and Associated CTs of Periosteum (Bone), Perichondrium (Cartilage), Adipose Tissue, Ligaments, Tendons, Blood Vessels, Blood, Bone Marrow, Trachea, Lungs, Esophagus, Stomach, Liver, Intestines, Spleen, Brain, Pancreas, Kidney, Urinary Bladder, Meninges, Testis, Tongue, and Thyroid, Thus Far
Species, M, Mouse; Rt, Rat; Rb, Rabbit; Ct, Cat; D, Dog; S, Sheep; G, Goat; P, Pig; Cw, Cow; Ho, Horse; Hu, Human; Clones Derived by Serial Dilution Single Cell Clonogenic Analysis; Rt-My, Rat Myoblast PC; Rt-Adip, Rat Adipoblast PC; RT-Chon, Rat Chondroblast PC; RT-Os, Rat Osteoblast PC; Con Hib, Contact Inhibited at Confluence Forming a Single Layer of cells; Sus, Suspension Cultures; Adh, Adherent To Type-I Collagen; No GF, No Growth Factors Added to Serum-Free Defined Medium; Inhib, Inhibitory Factors; LIF ADF, Prolif, Proliferation Factor, PDGF-BB to Stimulate Proliferation; Proliferation Assayed By Amount of DNA Per Well; Prolif Rate, Proliferation Rate During Log Phase Growth; Ds – Ws, Days To Weeks; Progre, Progression Agent Accelerates Phenotypic Expression of Lineage-Committed Progenitor
Cells; Induc, Induction Agent, General or Specific Induction Agents Used to Induce Multiple Phenotypes in Cultures; Commit, Commitment into Specific Cell; Diff Cs, Terminally Differentiated Cells; # Cs ID, Number of Cell Types Identified Using Limited Number of Cell Specific Assays in Laboratory; NA, Not Applicable; 3+Spermato, 3 + Spermatogonia; Pop Dbl, Population Doublings; Con DMSO, Concentration of Ultra-Pure Dimethylsulfoxide; # Cryo, Number Of Cells Cryopreserved for Optimum Cellular Recovery; B, Billion; M, Million; Op Fr & St T, Optimum Freezing & Storage Temperature; Fr Pro, Freezing Process, Rate at Which the Temperature was Reduced during Freezing; Thaw Pr, Thawing Procedure; Thaw T, Thawing Temperature; Karyo, Karyotypic Analysis; Telom, Telomerase Enzyme; Sonichh, Sonic Hedgehog; Cell Surface Markers Expressed, CD (cluster of differentiation) Markers and Cell Surface antigens expressed as assessed by Flow analysis and immunocytochemistry, CD1a, CD2, CD3, CD4, CD5, CD7, CD8, CD9, CD10, CD11b,
CD11c, CD13, CD14, CD15, CD16, CD18, CD19, CD20, CD22, CD23, CD24, CD25, CD31, CD33, CD34, CD36, CD38, CD41, CD42b, CD45, CD49d, CD55, CD56, CD57, CD59, CD61, CD62e, CD65, CD66e, CD68, CD69, CD71, CD79, CD83, CD90, CD95, CD105, CD117, CD123, CD135, CD166, Glycophorin-A, MHC-I,
HLA-DR-II, FMC-7, Annexin-V and Lin, CEA-CAM-1, SSEA, and Thy-1.
Animal repair models and human clinical trials, SM, Skeletal Muscle; C, cartilage; BV, blood vessels; IST, Inhibit Scar Tissue; PD, Parkinson disease; MI, Myocardial Infarction; PI, Pancreatic Islet Formation; B, Bone; AD, Alzheimer’s disease; MS, Multiple Sclerosis; ALS, Amyotrophic Lateral Sclerosis; CIDP, Chronic inflammatory demyelinating polyneuropathy; CP, Cerebral Palsy; TBI, Traumatic Brain Injury; Strk, Stroke; MD, Muscular Dystrophy; LI, Limb Ischemia; SLE, Systemic lupus erythematosus; CVD, Cardiovascular Disease; MI, Myocardial Infarction; BMT, Bone Marrow Transplant; FR, Facial reconstruction; IPF, Interstitial pulmonary fibrosis; ILD, interstitial lung disease; COPD, Chronic obstructive pulmonary disease; Asth, Asthma; TD, Thyroid Disease; Diab, Diabetes; LF, Liver Fibrosis; HL, Hair Loss; W, Wrinkles; Ag, Aging; OI, Orthopedic Injuries
|
Ch1 |
Sa2 |
Av3 |
Mo4 |
Rt5 |
Rb6 |
Fe7 |
Cn8 |
Ov9 |
Cp10 |
Pr11 |
Bo12 |
Eq13 |
HM14 |
HF15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Pre16 |
|
+17 |
|
|
|
|
|
|
|
|
|
|
+ |
+ |
|
Mor18 |
|
|
|
|
|
|
|
|
|
|
|
|
SEM19 |
|
|
SkM20 |
|
I21 |
|
|
|
|
|
|
|
|
|
|
I |
I |
|
Der22 |
|
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
Hrt23 |
|
I |
|
|
|
|
|
|
|
|
|
|
|
|
|
Psn24 |
+ |
|
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
+ |
|
Nb25 |
|
|
I |
I |
|
|
|
|
|
|
|
|
I |
I |
|
Ad26 |
|
|
I |
I |
|
|
|
|
|
|
|
|
|
I |
|
SM27 |
H,Hc28 |
|
I |
I |
I |
I |
I |
I |
I |
I |
I |
I |
I |
I |
|
Ge29 |
|
|
I |
|
|
|
|
|
|
|
|
I30 |
I31 |
I32 |
|
SkM |
H,Hc |
|
I,Cr33 |
I,Cr |
I |
I |
I |
I |
I |
I,Cr |
I |
I |
I |
I |
|
Der |
H,Hc |
|
I |
I,Cr |
|
|
|
|
|
I,Cr |
|
|
I |
I |
|
Hrt |
|
|
I |
I,Cr |
|
|
|
|
|
I,Cr |
|
|
|
|
|
GT34 |
|
|
|
I |
|
|
|
|
|
|
|
|
I |
I |
|
Pos35 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Pch36 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Ns37 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Adip38 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Lig39 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Ten40 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Bv41 |
H,Hc |
I |
I |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
BoM42 |
H,Hc |
I |
I,Cr |
I,Cr |
|
|
|
|
|
Cr |
|
|
I |
I |
|
Bld43 |
|
|
I |
I |
I |
I |
I |
I |
I |
I |
|
I |
I |
I |
|
Tra44 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Lng45 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Eso46 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Stm47 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Liv48 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
SmI49 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
LgI50 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Spl51 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Brn52 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Men53 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
SpC54 |
|
|
|
|
|
|
|
|
|
Cr |
|
|
|
|
|
Pan55 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Kid56 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Ub57 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Thy58 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
|
|
|
Tng59 |
|
|
|
I,Cr |
|
|
|
|
|
Cr |
|
|
Cr |
Cr |
|
Tes60 |
|
|
|
Cr |
|
|
|
|
|
|
|
|
|
|
|
FT61 |
|
|
|
Cr |
|
|
|
|
|
|
|
|
|
|
|
Kar62 |
|
|
Dip63 |
Dip |
|
|
|
|
|
Dip |
|
Dip |
Dip |
Dip |
Table 2 Species, Age and Location of Precursor Cells
Ch, Characteristic; Sa, Adult Terrestrial Salamanders, Ambystoma Annulatum, Ambystoma Maculatum, Ambystoma Texanum, Ambystoma Tigranum; Av, Avian, Gallus Domesticates; Mo, Mouse, Balb-C, CBF-1; Rt, Rat, Out Bred Sprague Dawley, in-bred Wistarfurth; Rb, Rabbit; Fe, Feline (cat); Cn, Canine
(dog); Ov, Ovine (sheep); Cp, Caprine (goat); Pr, Porcine (pig); Bo, Bovine (cow); Eq, Equine (horse); HM, Human Male; HF, Human Female; Pre,
Prenatal (before birth); +, Presence; Mor, Morula; SEM, Scanning Electron Microscopy; SkM, Skeletal Muscle; I, Isolation From The Tissues; Der, Dermis
of the Skin, Hrt, Heart; Psn, Post-Natal (after birth); Nb, Newborn; Ad, Adolescent; SM, Sexually Mature; Hc, Histology and Histochemistry; Ge, Geriatric;
Isolation from a 40 Year Old Horse; Isolation from a 67 Year Old Human Male; Isolation from a 87 Year Old Type-I Diabetic Female; Cr, Cryosectioned and
Immuostained with Carcinoembryonic Antigen-Cell Adhesion Molecule (CEA-CAM-1) for Totipotent Stem Cells and Stage Specific Embryonic Antigen
(SSEA) for Pluripotent Stem Cells; GT, Granulation Tissue; Pos, Periosteum; Pch, Perichondrium; Ns, Nerve Sheaths; Adip, Adipose Tissue (fat); Lig,
Ligament; Ten, Tendon; BV, Blood Vessels; BoM, Bone Marrow (Hematopoietic Cells and Stromal Cells); Bld, Blood; Tra, Trachea; Lng, Lung; Eso,
Esophagus (Lamina Propria, Submucosa, Adventitia); Stm, Stomach (Submucosa, Serosa); Liv, liver; SmI, Small Intestine (Lamina Propria, Submucosa,
Serosa); LgI, Large Intestine (Lamina Propria, Submucosam, Mesocolon); Spl, Spleen (Capsule, Trabeculae, Interstitial Tissue); Brn, Brain (White Mater,
Gray Matter); Men, Meninges (Dura Mater, Arachnoid Mater, Pia Mater); SpC, Spinal Cord (White Mater, Gray Matter); Pan, Pancreas (Exocrine And
Endocrine Portions); Kid, Kidney (Capsule And Interstitium); Ub, Urinary Bladder; Thy, Thyroid; Tng, Tongue; Tes, Testis; FT, Fallopian Tube; Kar,
Karyotype; Dip, Diploid Number of Chromosomes
|
Char1 |
BLSC2 |
ELSC3 |
GLSC4 |
EctoSC5 |
EndoSC6 |
MesoSC7 |
MesoProg8 |
|
CEA-CAM |
+ |
- |
- |
- |
- |
- |
- |
|
HCEA |
+ |
- |
- |
- |
- |
- |
- |
|
CEA |
+ |
- |
- |
- |
- |
- |
- |
|
CD66e |
+ |
- |
- |
- |
- |
- |
- |
|
DH-TuAg1 |
+ |
- |
- |
- |
- |
- |
- |
|
MC-480 |
+ |
+ |
- |
- |
- |
- |
- |
|
MC-631 |
+ |
+ |
- |
- |
- |
- |
- |
|
MC-813 |
+ |
+ |
- |
- |
- |
- |
- |
|
AlkPhos |
+ |
+ |
- |
- |
- |
- |
- |
|
CD66e |
+ |
- |
- |
- |
- |
- |
- |
|
CD10 |
- |
+ |
- |
- |
- |
- |
- |
|
CD90 |
- |
- |
+ |
+ |
+ |
+ |
- |
|
CD13 |
- |
- |
- |
- |
- |
+ |
- |
|
CD56 |
- |
- |
- |
+ |
- |
- |
- |
|
Pax-6 |
- |
- |
- |
+ |
- |
- |
- |
|
FORSE-1 |
- |
- |
- |
+ |
- |
- |
- |
|
Vimentin |
- |
- |
- |
+ |
- |
- |
- |
|
Nestin |
- |
- |
- |
+ |
- |
- |
- |
|
R401 |
- |
- |
- |
+ |
- |
- |
- |
|
HNES |
- |
- |
- |
+ |
- |
- |
- |
|
MAB353 |
- |
- |
- |
+ |
- |
- |
- |
|
RT-97 |
- |
- |
- |
+ |
- |
- |
- |
|
NF68 |
- |
- |
- |
+ |
- |
- |
- |
|
S-100 |
- |
- |
- |
+ |
- |
- |
- |
|
NF145 |
- |
- |
- |
+ |
- |
- |
- |
|
N-200 |
- |
- |
- |
+ |
- |
- |
- |
|
8A2 |
- |
- |
- |
+ |
- |
- |
- |
|
NG2 |
- |
- |
- |
+ |
- |
- |
- |
|
TH |
- |
- |
- |
+ |
- |
- |
- |
|
DOPA |
- |
- |
- |
+ |
- |
- |
- |
|
SV2 |
- |
- |
- |
+ |
- |
- |
- |
|
T8660 |
- |
- |
- |
+ |
- |
- |
- |
|
Tuj1 |
- |
- |
- |
+ |
- |
- |
- |
|
GFAP |
- |
- |
- |
+ |
- |
- |
- |
|
CNPase |
- |
- |
- |
+ |
- |
- |
- |
|
Rip |
- |
- |
- |
+ |
- |
- |
- |
|
MOSP |
- |
- |
- |
+ |
- |
- |
- |
|
MAB |
- |
- |
- |
+ |
- |
- |
- |
|
40E-C |
- |
- |
- |
+ |
- |
- |
- |
|
CD13/CD90 |
- |
- |
- |
- |
- |
+ |
- |
|
OP-137 |
- |
- |
- |
- |
- |
+ |
+ |
|
F5D |
- |
- |
- |
- |
- |
+ |
+ |
|
MF-20 |
- |
- |
- |
- |
- |
+ |
+ |
|
MY-32 |
- |
- |
- |
- |
- |
+ |
+ |
|
ALD58 |
- |
- |
- |
- |
- |
+ |
+ |
|
A4.74 |
- |
- |
- |
- |
- |
+ |
+ |
|
IA4 |
- |
- |
- |
- |
- |
+ |
+ |
|
Calp |
- |
- |
- |
- |
- |
+ |
+ |
|
MAB-3252 |
- |
- |
- |
- |
- |
+ |
+ |
|
MAB1548 |
- |
- |
- |
- |
- |
+ |
+ |
|
WV1D1 |
- |
- |
- |
- |
- |
+ |
+ |
|
MP111 |
- |
- |
- |
- |
- |
+ |
+ |
|
Von Kossa |
- |
- |
- |
- |
- |
+ |
+ |
|
CIIC1 |
- |
- |
- |
- |
- |
+ |
+ |
|
II-4CII |
- |
- |
- |
- |
- |
+ |
+ |
|
HC-II |
- |
- |
- |
- |
- |
+ |
+ |
|
Alcian Blue |
- |
- |
- |
- |
- |
+ |
+ |
|
AB 1.0 |
- |
- |
- |
- |
- |
+ |
+ |
|
AB 2.5 |
- |
- |
- |
- |
- |
+ |
+ |
|
Alcec Blue |
- |
- |
- |
- |
- |
+ |
+ |
|
AcB 1.0 |
- |
- |
- |
- |
- |
+ |
+ |
|
AcB 2.5 |
- |
- |
- |
- |
- |
+ |
+ |
|
Safranin-O |
- |
- |
- |
- |
- |
+ |
+ |
|
SO 1.0 |
- |
- |
- |
- |
- |
+ |
+ |
|
SO 2.5 |
- |
- |
- |
- |
- |
+ |
+ |
|
HC-II |
- |
- |
- |
- |
- |
+ |
+ |
|
D1-9 |
- |
- |
- |
- |
- |
+ |
+ |
|
9/30 |
- |
- |
- |
- |
- |
+ |
+ |
|
12/21 |
- |
- |
- |
- |
- |
+ |
+ |
|
12C5 |
- |
- |
- |
- |
- |
+ |
+ |
|
H-CD34 |
- |
- |
- |
- |
- |
+ |
+ |
|
CD31 |
- |
- |
- |
- |
- |
+ |
+ |
|
P1H12 |
- |
- |
- |
- |
- |
+ |
+ |
|
P2B1 |
- |
- |
- |
- |
- |
+ |
+ |
|
P8B1 |
- |
- |
- |
- |
- |
+ |
+ |
|
P2H3 |
- |
- |
- |
- |
- |
+ |
+ |
|
H-endo |
- |
- |
- |
- |
- |
+ |
+ |
|
H5A4 |
- |
- |
- |
- |
- |
+ |
+ |
|
Hermes-1 |
- |
- |
- |
- |
- |
+ |
+ |
|
H5A5 |
- |
- |
- |
- |
- |
+ |
+ |
|
H5C6 |
- |
- |
- |
- |
- |
+ |
+ |
|
1B10 |
- |
- |
- |
- |
- |
+ |
+ |
|
SudBlk-B |
- |
- |
- |
- |
- |
+ |
+ |
|
Oil Red-O |
- |
- |
- |
- |
- |
+ |
+ |
|
H-AFP |
- |
- |
- |
- |
+ |
- |
- |
|
DESMO |
- |
- |
- |
- |
+ |
- |
- |
|
LAP |
- |
- |
- |
- |
+ |
- |
- |
|
151-Ig |
- |
- |
- |
- |
+ |
- |
- |
|
H4Ac19 |
- |
- |
- |
- |
+ |
- |
- |
|
OC2 |
- |
- |
- |
- |
+ |
- |
- |
|
OC3 |
- |
- |
- |
- |
+ |
- |
- |
|
OC4 |
- |
- |
- |
- |
+ |
- |
- |
|
OC5 |
- |
- |
- |
- |
+ |
- |
- |
|
OC10 |
- |
- |
- |
- |
+ |
- |
- |
|
H.4 |
- |
- |
- |
- |
+ |
- |
- |
|
H.1 |
- |
- |
- |
- |
+ |
- |
- |
|
DPPIV |
- |
- |
- |
- |
+ |
- |
- |
|
OV6 |
- |
- |
- |
- |
+ |
- |
- |
|
HESA |
- |
- |
- |
- |
+ |
- |
- |
|
cells |
- |
- |
- |
- |
+ |
- |
- |
|
YM-PSO87 |
- |
- |
- |
- |
+ |
- |
- |
|
CELLS |
- |
- |
- |
- |
+ |
- |
- |
|
YM-PS5088 |
- |
- |
- |
- |
+ |
- |
- |
|
cells |
- |
- |
- |
- |
+ |
- |
- |
|
11180 |
- |
- |
- |
- |
+ |
- |
- |
|
CK-19 |
- |
- |
- |
- |
+ |
- |
- |
Table 3 Staining Characteristics of Endogenous Stem Cells and their Induced Cell Types
Char, Characteristics Tested; BLSC, Blastomere-Like Stem Cells;9,31 ELSC, Epiblast-Like Stem Cells;10,31 GLSC, Germ Layer Lineage Stem Cells;31 EctoSC, Germ Layer Ectodermal Stem Cells;10,31 EndoSC, Germ Layer Endodermal Stem Cells;10,31 MesoSC, Germ Layer Mesodermal Stem Cells;10,11,31 MesoProg, Multipotent Mesenchymal Progenitor Cells31
Progenitor cells
By definition lineage/tissue/cell-committed progenitor cells do not demonstrate inherent plasticity, but rather have both a defined differentiation potential (Figure 1 & 3) (Table 3) and a limited replicative lifespan. For example, unipotent myosatellite myoblast progenitor cells will only form skeletal muscle;34 bipotent adipo-fibroblast progenitor cells will only form adipocytes (fat cells) and fibroblasts;35,36 tripotent chondroblast-osteoblast-adipoblast progenitor cells will only form cartilage, bone and fat cells;37–39 while multipotent hematopoietic progenitor cells will form the myriad of cell types within the hematopoietic lineage but not any cell type outside that lineage.40,41 Progenitor cells also display a limited population doubling lifespan before pre-programmed senescence and cell death occurs. This lifespan has been defined by the absolute number of population doublings, and has been reported as 8-10 population doublings for rodents42 and 50-70 population doublings for humans.43 This “biological clock” defined by population doublings begins at the birth of the individual.
Endogenous stem cells
In contrast to adult-derived lineage/tissues/cell-committed progenitor cells, adult-derived lineage-uncommitted stem cells demonstrate an inherent plasticity, being capable of replicating themselves and forming their respective downstream cell types (Figure 1 & 3) (Table 4).31 Unlike embryonic stem cells (that are preprogrammed to spontaneously differentiate into tissues of the embryo/fetus25 or lineage-committed progenitor cells (that are lineage-committed to be multipotent, tripotent, bipotent or unipotent committed cells 34–41), uncommitted adult stem cells are not preprogrammed to form anything. Rather, they respond to environmental cues (i.e., inductive agents) to differentiate into specific cell types based on the particular activity of an inductive agent. For example, nerve growth factor induces totipotent stem cells, pluripotent stem cells and ectodermal stem cells to form neurons; bone morphogenetic protein-2 induces totipotent stem cells, pluripotent stem cells and mesodermal stem cells to form bone; while hepatocyte growth factor induces totipotent stem cells, pluripotent stem cells and endodermal stem cells to form hepatocytes.10,31,33–44
|
Char1 |
BLSCs2
|
Tr-BLSC/ ELSCs3 |
ELSCs4
|
Tr-GLSCs5 |
MesoSCs6 |
MesPCs7
|
|
Sizem |
0.2-2 |
2-6 |
6-7 |
7-8 |
8-10 |
10-20 |
|
Trypan blue |
Pos8 |
Pos/Neg |
Neg9 |
Neg |
Neg |
Neg |
|
Days Viab PM10 |
30+ |
30+ |
7+ |
5 |
3 |
1 |
|
Viab T11 |
4oC |
4oC |
4oC |
4oC |
4oC |
4oC |
|
Tiss12 |
Yes |
Yes |
Yes |
Yes |
Yes |
Yes |
|
Species13 |
M,Rt,Rb, Ct,D,S, G,P,Cw, Ho,Hu |
M,Rt,Rb, Ct,D,S, G,P,Cw, Ho,Hu |
M,Rt,Rb, Ct,D,S, G,P,Cw, Ho,Hu |
Hu |
M,Rt,Rb, Ct,D,S, G,P,Cw, Ho,Hu |
M,Rt,Rb, Ct,D,S, G,P,Cw, Ho,Hu |
|
Clones14 |
BLSC Scl-44 BLSC Scl-9 |
NYD |
ELSC Scl-40 |
NYD |
MSC Scl-2PG |
Rt-My15 Rt-Adip16 Rt-Chon17 Rt-Os18 |
|
Con Hib19 |
No |
No |
No |
No |
Yes |
Yes |
|
Growth |
Adherent |
Adherent |
Adherent |
Adherent |
Adherent |
|
|
No GF22 |
Quiescent |
Quiescent |
Quiescent |
Quiescent |
Quiescent |
Quiescent |
|
Inhib23 |
Respond |
Respond |
Respond |
Respond |
Respond |
Respond |
|
Prolif24 |
Prolif25 |
Prolif |
Prolif |
Prolif |
Prolif |
Prolif |
|
Prolif Rate26 |
12-14 hr |
12-14 hr |
12-14 hr |
14-18 hr |
18-24 hr |
Ds – Ws27 |
|
Progre28 |
No |
No |
No |
No |
No |
Yes |
|
Induc29 |
Yes |
Yes |
Yes |
Yes |
Yes |
No |
|
Commit30 |
Tr-BLSC/ELSCs |
ELSCs |
Tr-GLSCs |
MesoSCs |
MesoPCs |
Diff Cs31 |
|
# Cs ID32 |
68 |
67 |
63 |
62 |
39 |
NA33 |
|
Lineages |
3+Spermato34 |
3 |
3 |
3 |
1 |
1 |
|
Pop Dbl35 |
>300 |
>300 |
>400 |
>400 |
>690 |
50-70 |
|
Con DMSO36 |
7.5% v/v |
7.5% v/v |
7.5% v/v |
7.5% v/v |
7.5% v/v |
10% |
|
# Cryo37 |
1-10 B38 |
1-10 B |
1-10 M39 |
1-10 M |
1-10 M |
1-10 M |
|
Op Fr & St T40 |
-80oC |
-80oC |
-80oC |
-80oC |
-70oC |
-196oC |
|
Fr Pro41 |
Slow |
Slow |
Slow |
Slow |
Slow |
Flash |
|
Thaw Pr42 |
Fast |
Fast |
Fast |
Fast |
Fast |
Fast |
|
Thaw T43 |
37oC |
37oC |
37oC |
37oC |
37oC |
37oC |
|
Recovery |
>98% |
>98% |
>98% |
>98% |
>98% |
>95% |
|
Karyo44 |
Normal |
Normal |
Normal |
NYD |
Normal |
Normal |
|
Genes Expressed |
Telom45 Bcl-2, Nanog, Nanos, CXCR4 |
NYD |
Telom, Oct-4, SSEA, Sonic hh46 |
NYD |
Telom |
NYD |
|
Cell Surface Markers Expressed47 |
CD66e, CEA |
CD66e, CEA, CD10, SSEA |
CD10, SSEA |
CD10, SSEA, CD90, Thy-1 |
CD90, Thy-1, CD13, MHC-I |
CD105, CD117, CD166, MHC-I |
|
Animal48
|
SM, C, BV, IST, PD, MI, PI |
SM, C, BV, IST, PD, MI, PI |
SM, C, BV, IST, PD, MI, PI |
SM, C, BV, IST, PD, MI, PI |
SM, C,B, BV, IST
|
SM, C,B, BV, IST
|
|
Human48 |
PD, AD, MS, ALS, CIDP, CP, TBI, Strk, Sciatica, MD, LI, SLE, CVD, MI, BMT, FR, IPF, ILD, COPD, Asth, TD, Diab, LF, HL, W, Ag, OI |
PD, AD, MS, ALS, CIDP, CP, TBI, Strk, Sciatica, MD, LI, SLE, CVD, MI, FR, IPF, ILD, COPD, Asth, TD, Diab, LF, HL, W, Ag, OI |
PD, AD, MS, ALS, CIDP, CP, TBI, Strk, Sciatica, MD, LI, SLE, CVD, MI, FR, IPF, ILD, COPD, Asth, TD, Diab, LF, HL, W, Ag, OI |
PD, AD, MS, ALS, CIDP, CP, TBI, Strk, Sciatica, MD, LI, SLE, CVD, MI, FR, IPF, ILD, COPD, Asth, TD, Diab, LF, HL, W, Ag, OI |
MD, LI, SLE, CVD, FR, W, Ag, OI, MskI,
|
NYD |
Table 4 Adult Precursor Cell Characteristics
Totipotent stem cells will replicate themselves as well as forming similar downstream cell types, similar to embryonic blastomeres of the morula stage of development. Thus, they will form all somatic tissues within the embryo proper including spermatogonia (Figure 1).9,31,45 They are equivalent in differentiation potential to the blastomeres of the morula and blastocyst. Pluripotent stem cells will replicate themselves and form similar downstream cell types as embryonic epiblast cells (all somatic cells of the embryo but not the gametes) (Figure 1)1,9,10,31,33,46–48 and are thus equivalent in differentiation potential to the cells of the epiblast. Germ layer lineage stem cells (ectodermal, mesodermal and endodermal stem cells) will replicate themselves as well as form downstream cell types belonging to their respective germ layer lineages (Figure 1).10,11,31,33 They are thus equivalent in differentiation potential to the cells comprising the ectodermal, mesodermal, and endodermal germ layer lineages, respectively. Precursor cells that are “true” stem cells and not misnamed progenitor cells (lineage-uncommitted cells rather than lineage-committed cells) contain the telomerase enzyme as long as the cells remain lineage-uncommitted (Figure 5) (Table 4).10,31,33 The telomerase enzyme adds telomeres to the ends of the chromosomes,49 protecting the chromosomes from degradation due to increased mitotic divisions. Protection from chromosomal degradation gives these postnatal stem cells the capability for extensive proliferation. The preprogrammed biological clock for endogenous stem cells does not begin at birth of the individual, but rather when the stem cells commit to a particular progenitor tissue/cell lineage.9,10,31 Although it has been reported that endogenous pluripotent stem cells do not exist,50 a sufficient number of separate laboratories have proven that endogenous pluripotent stem cells do exist and can be readily isolated, characterized and utilized.
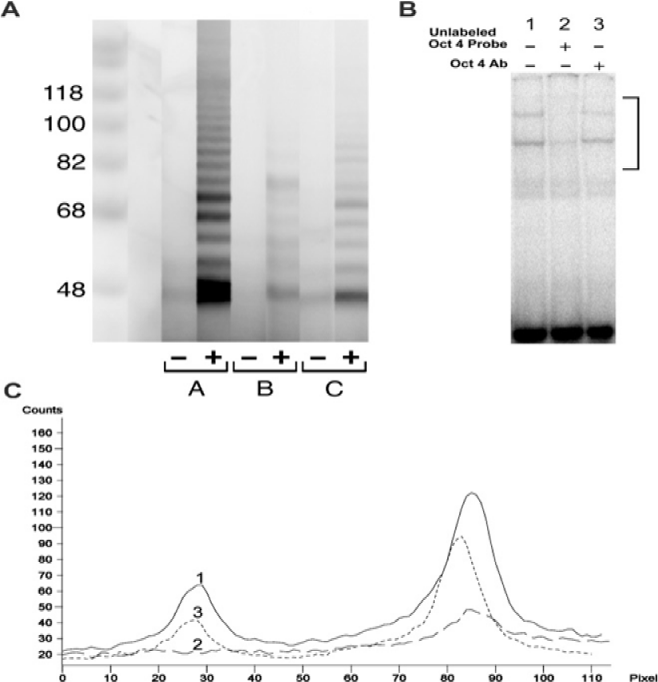
Figure 5 Molecular analysis of telomerase activities.
Figure 5A: in an adult rat mesodermal stem cell clone, MSC-Scl-2PG, and an adult rat Pluripotent stem cell clone, ELSC-Scl-40 and Oct-4 gene expression.
Figure 5B & 5C: ELSC-Scl-40.
A. Telomerase activity was detected by PAGE of cell lysates from MSC-Scl-2PG at 151 population doublings and ELSC-Scl-40 at 254 population doublings. Cells were processed as described (TRAPeze Assay, Integrin). Lane A -, extraction buffer only (controls); Lane A +, extract of telomerase-positive cells; lane B -, heat inactivated extract of MSC-Scl-2PG; lane B +, test extract of MSC-Scl-2PG; lane C -, heat inactivated extract of ELSC-Scl-40 lane 2 +, test extract of ELSC-Scl-40 Note presence of a laddering of bands denoting the presence of telomerase activity; compare lanes 1, 2 and 3.
B. Oct-4 gene was detected in ELSC-Scl-40 by the electrophoretic mobility shift assay using the oligonucleotide 5’-TGTCGAATGCAAATCACTAGA-3’ containing the Oct-1 consensus binding site. Two bands that represent binding by members of the Oct family of transcription factors were obtained, shown by competition for binding by unlabeled Oct oligonucleotide.
C. Densitometric analysis of the area contained in the side bar of (Figure B). Lane 1, solid line; lane 2, long dashes; lane 3, short dashes. Incubation with Oct-4-specific antibody substantially decreased the formation of the lower band, indicating the presence of Oct-4 gene expression.
Amniotic fluid-derived pluripotent stem cells
The amniotic fluid contains a mixture of predominantly epithelial cells. It is thought that they are sloughed off from the epidermis, gastrointestinal, and urinary epithelia. They range from six to fifty microns in size and express cell surface markers from all three primary germ layer lineages.51–54 Within this heterogeneous population are a small percentage of cells that display the c-kit cell surface marker by FACS analysis.55 This c-kit-positive population of cells were expanded to sub-confluence and passaged every 48-72 hours. These c-kit-positive cells derived from the amniotic fluid demonstrate greater than 300 population doublings, which is far greater than Hayflick’s limit.43 The cells were shown to have a normal karyotype, even at late passages, and express normal G1 and G2 checkpoints.55 These c-kit-positive cells express telomerase activity in late passages.56,57 They are positive for SSEA4 and Oct4 markers, but are negative for SSEA1, SSEA3, CD4, CD8, CD34, CD133, C-MET, ABCG2, NCAM, BMP4, TRA1-60 and TRA1-81.55 When implanted into immunodeficient mice, these cells do not form teratomas.25 When induced to differentiate utilizing various cocktails of inductive factors, these cells expressed phenotypic expression markers for differentiated cells from the three primary germ layer lineages (i.e., ectoderm, mesoderm, and endoderm). For example, utilizing RT-PCR analysis the following lineage-specific markers were expressed. For cells derived from the ectodermal lineage, neuronal and glial cells were induced. These cells expressed markers for βIII-tubulin, glutamic acid, and glial fibrillary acidic protein.58,59
Four cell types were induced corresponding to mesodermal derived cells (adipocytes) which expressed peroxisome proliferation-activated receptor gamma-2 (pparγ-2);60,61 endothelial cells, which expressed human-specific endothelial cell marker (P1H12), factor VIII (FVIII), kinase insert domain-containing receptor (KDR), platelet cell adhesion molecule 1 (PECAM1 or CD31), and vascular cell adhesion molecule (VCAM);55 myocytes, which expressed <em>Myf6, MyoD </em>, and desmin;62,63 and osteocytes, which expressed core-binding factor A1 and osteocalcin.64,65 Hepatocytes, demonstrating markers for transcription factor HNF-4α, c-met receptor, the MDR membrane transporter, albumin and α-fetoprotein, were induced.55 This suggested that the c-kit-positive amniotic fluid-derived cells could be induced into differentiated cells derived from the endodermal germ layer lineage. Lastly, the above results could be repeated in collections of c-kit positive cells from the amniotic fluid or from a population of c-kit-positive cells expanded from a single cell.55 These results suggested that the c-kit-positive amniotic fluid-derived cells are pluripotent in their differentiation capabilities.
Multipotent Adult Progenitor Cells (MAPCs)
Multipotent adult progenitor cells (MAPCs) were isolated from the bone marrow, muscle and brain of mice by Reyes M et al.66,67 Originally, these stem cells were designated as mesenchymal because they appeared to resemble those described by Caplan.68 MAPCs were later demonstrated to form neurons (ectodermal germ layer lineage),69–71 and hepatocytes (endodermal germ layer lineage).72,73 Thus, MAPCs can differentiate into cell types from all three primary germ layer lineages. Thus MAPCs appear to be pluripotent stem cells rather than mesenchymal stem cells as they were first designated. Along the stem cell developmental continuum from totipotent stem cells (which grow in suspension culture to adherent culture) to unipotent progenitor cells (which grow as adherent cultures) (Table 4), MAPCs grow as adherent cultures.
Very small embryonic-like stem cells
Very small three to five micron cells were originally isolated from adult murine bone marrow (Sca1+Lin- CD45-),74–76 and human cord blood (CD133+Lin-CD45-).77–79 These cells were detectable with early developmental markers, such as cell surface SSEA, and nuclear Oct4 and Nanog transcription factors. These particular cells have a high nuclear to cytoplasmic ratio and demonstrate a preponderance of euchromatin rather than heterochromatin. Cells with these same characteristics have been found in skeletal muscle,80 gonads,81 heart77,81 and brain.77 In the appropriate in vivo model systems, these cells have been shown to differentiate into long-term repopulating mesenchymal stem cells,80 skeletal muscle,81 endothelial cells,80,82 cardiac myocytes,75 hematopoietic stem cells,83 lung epithelial cells,84 tumor stromal cells85 and oocytes.86 Because of their small size, embryonic markers, and ability to form multiple primitive cell layers of the conceptus, these cells have been designated as very small embryonic-like stem cells (VSELs).87 Very low numbers of VSELs circulate throughout the vasculature under steady-state conditions.87 Additional VSELs can be mobilized in patients during myocardial infarction,88 stroke,89 acute burns,90 active inflammatory bowel disease,91 and cancer92 and with the use of granulocyte-colony stimulating factor.93
Adult Pluripotent Stem Cells
Six to eight micron cells (SSEA+/CD10+),9,10,31–33 and 0.2 to 2.0 micron cells (CEA-CAM-1+ / CD66e+)9,31 were originally isolated from mouse, rat and human skeletal muscle of newborn, adolescent, sexually mature and geriatric-aged individuals. These cells were detectable with early developmental markers, such as cell surface SSEA (stage specific embryonic antigen)10,31,44 and CEA-CAM-1 (carcinoembryonic antigen-cell adhesion molecule-1),10,33,46 nuclear transcription factors Nanog,10,31,44 Nanos, Bcl-2, CXCR4, Oct4, and telomerase (Figure 5) (Table 4).9,31,44 These particular cells have a high nuclear to cytoplasmic ratio. These cells display a normal karyotype after population doublings well in excess Hayflick’s limit.45,94 Cells with these same characteristics have been found in 37 different tissues and organs thus far (Table 2), including blood, bone marrow, brain, adipose tissue, and skeletal muscle. In the appropriate in vivo and in vitro model systems these cells have been shown to differentiate and demonstrate phenotypic expression markers for 63 (6-8 µm cells) and 66 (0.2-2.0 µm cells) distinct cell types, including neurons, glial cells, keratinocytes, muscle, fat, cartilage, bone, connective tissues, hematopoietic cells, GI epithelium, liver cells, pancreatic cells, and spermatogonia (Table 3).9,10,31,33,44 Because of their small size, presence of embryonic markers and their ability to form cells from the three primary germ layer lineages as well as spermatogonia, these cells were named according to parallel structures found within the developing zygote, i.e., epiblast-like stem cells (ELSCs, 6-8 µm cells)10 and blastomere-like stem cells (BLSCs, 0.2-2.0 µm cells).9 Moderate numbers of these cells circulate throughout the peripheral vasculature under steady state conditions.31,95–97 Additional ELSCs and BLSCs can be mobilized and harvested following exercise,97 severe trauma96 and after ingestion of a nutraceutical.31,95,97
Additional Pluripotent Stem Cells
Following an isolation and propagation protocol similar to that used for the MAPCs, a cell termed “unrestricted somatic stem cell (USSC)” was isolated from bone marrow in 1-3 weeks, but with only three cell passages. The cells showed the capability to form ectodermal, mesodermal and endodermal phenotypes.98 Marrow-isolated adult multilineage inducible (MIAMI) cells were also derived in a manner similar to MAPCs.99 Their derivation is based on seeding densities at either clonal or sparse dilutions. MIAMI cells demonstrated differentiation into ectodermal, mesodermal and endodermal phenotypes.100 Human bone marrow-derived multipotent stem cells (hBMSCs) have been reported.101 As the name implies, these cells were isolated from human bone marrow and selected from adherent cultures. Differentiation studies showed that these cells form phenotypes belonging to all three germ layer lineages. Fetal somatic stem cells (FSSCs) have been reported.102 The cells, derived from fetal soma, demonstrate a wide range of differentiation potentials.
Reprogrammed Pluripotent Stem Cells
The idea of cloning animals utilizing nuclear transfer was first proposed by Spemann.103 The first demonstration that Spemann’s proposal was even possible for cloning adult animals was shown by Gurdon.104 Indeed, the concept of reprogramming a patient’s somatic cells into totipotent/ pluripotent stem cells was conceived based on four independent breakthroughs in the field of developmental embryology in the late 1900’s, i.e., somatic cell nuclear transfer in amphibians;104 success of cloning of sheep (Dolly) by somatic cell nuclear transfer;105,106 assisted reproductive technologies for live human births (Mary Louise Brown);12 and derivation of human embryonic stem cells.25,26
Somatic Cell Nuclear Transfer (SCNT)
The process of somatic cell nuclear transfer, i.e., reprogramming of somatic cell nuclei into a totipotent embryonic stem cell capable of forming an organism, was first proposed by Hans Spemann when he referred to a “fantastical experiment” in his book, <em>Embryonic Development and Induction</em>.103 However, Spemann’s process was first demonstrated by Gurdon.104 He utilized the nuclei from the intestinal epithelial cells of Xenopus laevistad poles and transferred the nuclei into the cytoplasm of enucleated eggs to demonstrate the development of an adult frog. His work demonstrated that the ova contained maternal factors within its cytoplasm with the inherent ability to reprogram gene expression of a differentiated somatic cell nucleus and that that nucleus had the capability of forming a new organism of the same genetic makeup as the host organism.107 His experiment represented the first reported example of a somatic cell being reprogrammed back to a totipotent state by an enucleated egg and developing into a live, viable offspring.108
Therapeutic cloning, or SCNT, begins with the same process used to create Dolly.106 A diploid donor somatic cell from a body tissue, such as a fibroblast from skin, is stripped of its plasma membrane and cytoplasm and fused with an enucleated unfertilized ovum. The cytoplasm within the ovum reprograms the DNA within the diploid donor nucleus to an embryonic state. The fused cell (modified blastomere) is allowed to rest for a defined length of time and then induced to proliferate until it reaches the early blastocyst stage, composed of the inner cell mass and trophoblast. The inner cell mass cells are harvested and cultured to create a stable cell line that is genetically matched to the donor cells. The cells are pluripotent in that they:
In this technique a nucleus from a differentiated somatic cell of the recipient replaces the nucleus of a donor blastomere. The modified blastomere is allowed to proliferate utilizing an inhibitor, the formation of embryoid bodies occurs, the inhibitor is removed, the embryoid bodies are allowed to spontaneously differentiate and only those cell types necessary for the transplant are utilized. Since the resultant embryonic stem cells contain the recipient’s nucleus, HLA-antigens are produced that match the recipient. Once transplanted, the cells/tissues are not rejected by the recipient.
While experiments using SCNT for the creation of animals including mammals continued, the majority reported using nuclei from pre-natal individuals. For example, Illmensee and Hoppe109 reported the formation of cloned mice utilizing the nuclei from pre-implantation embryos with oocyte cytoplasm. Willadsen110 reported the successful cloning of sheep and then cattle, using 8–16 cell embryos as nuclei donors. Sims and First111 reported the formation of cattle by SCNT where the nuclei were produced from cells of the inner cell mass that were cultured for up to 28 days under conditions that attempted to maintain the potency of the original cells. Utilizing a different approach, Campbell et al.105 grew embryo-derived cells for extended passages under standard tissue culture conditions, leading to a differentiated cell type. Using these differentiated cells for SCNT, viable offspring were produced, but only following serum starvation of the cells to induce a quiescent cell-cycle state. Wilmut et al.106 using adult mammary epithelial cells as the nucleus donors, reported the formation of Dolly, a sheep, which was the first cloned animal derived from an adult somatic cell.108 The process used to create Dolly has since been verified in mice,112 cattle,113,114 pigs,115 goats,116 rabbits,117 cats,118 mules,119 horses,120 rats121 and dogs.122
Recently, three laboratories reported the derivation of human embryonic stem cell lines utilizing therapeutic cloning/SCNT technologies. Tachibana et al.123 created human embryonic stem cell lines utilizing isolated somatic cell nuclei from fetal cells and from an 8-month old infant which were subsequently fused to enucleated oocytes. The fused cells were allowed to rest for 30 minutes before the initiation of cellular proliferation. The resultant embryonic stem cell lines carried the genomes from their respective hosts, exhibited spontaneous differentiation into all three primary germ layer lineages and could form teratomas.
Chung et al.124 reported fusing fibroblast nuclei from 35-year-old and 75-year-old males with enucleated oocytes. The fused eggs, containing the DNA of mature fibroblasts, were plated onto a feeder layer of mouse embryonic fibroblasts. They were stimulated to divide and within five to six days developed into blastocysts containing a pluripotent inner cell mass. From the inner cell mass they were able to generate karyotypically normal (46, XY) diploid human embryonic stem cell lines. The two hESCs lines expressed OCT-4, SSEA-4, TRA-1-60, and TRA-1-81, exhibited spontaneous differentiation into all three germ layers, and formed teratomas. This is of particular importance because the embryonic stem cells can be induced to form any tissues that the body might need for replacement and/or repair. One key point in their study was the two hour time window the fused eggs were allowed to “rest” before initiation of cellular proliferation. They proposed that this time window was crucial to initiate reprogramming of mature DNA in the egg prior to onset of cellular proliferation.
Yamada et al.125 reported the derivation of a human embryonic stem cell line utilizing the nuclei of somatic cells from the skin of a 32-year old woman with Type 1 diabetes mellitus. The embryonic stem cell line exhibited spontaneous differentiation into all three germ layers, and could form teratomas. The researchers also succeeded in differentiating these embryonic stem cells into insulin-producing cells. If SCNT technology to generate human embryos from adult differentiated cells is reproducible in multiple laboratories, it will permit the derivation of patient-specific embryonic stem cells for use in patient-specific cell therapy approaches. Unlike protocols necessary to generate iPSCs, SCNT does not require plasmids or viral reprogramming methods, and therefore is much safer for the overall outcome of the patient.
Induced Pluripotent Stem Cells (iPSCs)
The most widely accepted method to generate induced pluripotent stem cells (iPSCs) is the retroviral vector introduction of four genes (Oct4, Sox2, Klf4and c-Myc, a.k.a., the Yamanaka factors) into more differentiated cell types, such as fibroblasts.126 Following the initial landmark work for which Yamanaka received the 2013 Nobel prize, iPSCs have since been generated from differentiated fetal and adult fibroblasts;126–133 hepatocytes;134 stomach cells;135 Keratinocytes;136 cord blood;136–138 peripheral blood;139,140 fully differentiated B- and T- lymphocytes;141–146 dental pulp cells;147–149 and kidney cells.150 However, it has also been recognized that the less differentiated the cell type, the fewer the number of Yamanaka factors (genes) are required to induce pluripotency. More efficient reprogramming has been shown to occur in progenitor cells rather than terminally differentiated cells.151 For example, umbilical cord cells that already express Klf4 and c-Myc were found to form iPSCs when challenged with Oct4 and Sox2.138 Alternatively, neural progenitor cells, expressing the Sox2 gene could be induced to form IPSCs with the insertion of Oct4 and Klf4.152–154 Recent reports demonstrate that iPSCs can be generated from the granulosa cells of ovarian follicles by Oct4 and Sox2.151 Indeed, iPSCs demonstrate enhanced expression of the pluripotency-associated genes, Oct4, Telomerase (Figure 5), and SSEA,155,156 which are similar to the genes expressed in endogenous pluripotent stem cells.10,33,44,74,78,88
There are a number of endogenous and reprogrammed pluripotent cells that could be used for transplantation for various therapeutic purposes. Time will tell which ones become chosen for transplants to treat the many conditions that could benefit from such treatments.
None.
The author declares that there is no conflict of interest.

©2014 Young, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.