MOJ
eISSN: 2373-4442


Case Report Volume 2 Issue 2
1Division of Gastroenterology and Hepatology, Osaka Kaisei Hospital, Japan
2Laboratory of Host Defenses Institute for Advanced Medical Science, Hyogo College of Medicine, Japan
3Division of Gastroenterology and Hepatology, Osaka Hospital of Japan Community Healthcare Organization, Japan
Correspondence: Yutaka Kishida, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Osaka Kaisei Hospital, 1-6-10, Miyahara, Yodogawa-Ku, Osaka City, Osaka, 532-0003 Japan, Tel 81-6-6393-6234, Fax 81-75-955-3946
Received: February 03, 2015 | Published: April 23, 2015
Citation: Kishida Y, Imaizumi N, Tanimura H, Kashiwamura S, Kashiwagi T (2015) Treatment of Chronic Hepatitis C with Viral-Suppression linked to Restoration of Innate-Immune Responses with Induction-Therapy with n-IFN-beta followed by Simeprevir. MOJ Immunol 2(2): 00040. DOI: 10.15406/moji.2015.02.00040
Complications associated with hepatitis C virus (HCV) infection may be prevented by viral eradication. Response rates with direct–acting antivirals regimens are generally lower in chronic hepatitis C (CHC) patients who have failed to respond to previous interferon (IFN) treatments. HCV persistence in the host results from in efficient innate and adaptive immune responses. The resolution of a HCV infection may restore impairments in innate and adaptive immunities. Our previous study suggested that induction treatment with natural (n)–IFN–beta followed by maintenance treatment with n–IFN–alpha restored innate immune responses, as indicated by the significant increase of IL–12, and IL–15 and significant decrease of CXCL–8. Persistent virologic clearance and the restoration of innate immune responses with Simeprevir plus Pegylated (Peg) – IFN–alpha/ribavirin (RBV) were more likely to result in a sustained virologic response (SVR). On the basis of these findings, we conducted to treat a CHC patient with genotype 1b, a high viral load, a prior null response to IFN treatments and chronic active hepatitis with advanced fibrosis using induction therapy with n–IFN–beta followed by Simeprevir plus Peg–IFN–alpha–2b/RBV. This is the first case of difficult–to–treat CHC with genotype 1b, a high viral load, null response to previous IFN treatment and advanced hepatic fibrosis that was successfully treated with induction therapy using n–IFN–beta, which induced the viral clearance of serum HCV RNA, followed by Simeprevir plus Peg–IFN–alpha–2b/RBV. Persistent viral clearance linked to restoration of innate immune responses and SVR were achieved 12 weeks after cessation of the treatment without virologic breakthrough or relapse and sustained biochemical response. Anemia and thrombocytopenia were managed without discontinuation of the treatment. Induction therapy with n–IFN–beta followed by Simeprevir plus Peg–IFN–alpha–2b/RBV was tolerated well and only mild adverse effects were noted. It also effectively eradicated HCV infection in a difficult–to–treat CHC patient with advanced fibrosis.
Keywords: Chronic hepatitis C; Innate immune response; Interferon–beta; Simeprevir; Peg–IFN–alpha; Ribavirin
CHC: Chronic Hepatitis C; IFN: Interferon; HCV: Hepatitis C Virus; SVR: Sustained Virologic Response; RVR: Rapid Virologic Response; EVR: Early Virologic Response; cEVR: Complete Early Virologic Response; ETVR: End Treatment Virologic Response; DAAs: Direct Acting Antivirals; CPIT: Cyclic And Periodic Interferon Treatment; NCT: Novel Combination Treatment; SBR: Sustained Biochemical Response; AEs: Adverse Effects
170 million people are chronically infected with hepatitis C virus (HCV) worldwide. Chronic HCV infection leads to the development of cirrhosis, liver failure, liver cancer and liver transplantation. More than 350,000 die annually from liver diseases caused by HCV. The prevalence of HCV infection is estimated to be about 3 % in the world.1 HCV associated liver diseases may be prevented by viral eradication. Throughout the past decade, the therapy for chronic hepatitis C (CHC) patients has consisted of pegylated (Peg) interferon (IFN) alpha plus ribavirin (RBV). This treatment has produced rates of sustained virologic response (SVR) of 40–50% in HCV genotype–1 infected patients who are most prevalent in Europe, USA, Asia and Japan. A new era in the therapy of HCV has emerged with direct–acting antivirals (DAAs), which inhibit the HCV NS3/4A viral protease. The new standard therapy for HCV genotype 1 infected patients is triple therapy using a protease inhibitor plus Peg–IFN–alpha/RBV. Simeprevir is a HCV NS3/4A second–generation protease inhibitor for the treatment of HCV infection.
Triple therapy consisting of Simeprevir plus Peg–IFN–alpha/RBV led to higher SVR rates than Peg–IFN–alpha/RBV alone in treatment–naïve and –experienced CHC patients. SVR rates in CHC patients with triple therapy using Simeprevir were 38–59 % with a previous null response to Peg–IFN–alpha/RBV, 48–86% with a previous partial response, and approximately 77–89% with previous relapse.2 Response rates with DAAs regimens are generally lower in patients who have failed prior treatments with Peg–IFN–alpha than in treatment–naïve patients, and are markedly lower among previous null responders. Therefore, more effective therapies in patients with a previous null response to IFN treatments are required. The defect of an early virologic response (EVR) at week 12 of treatment is a negative predictor of SVR. On the contrary, achievement of a rapid virologic response (RVR) defined as clearance of serum HCV RNA at week 4 is a positive predictor of SVR. High rates of SVR 24 have been reported in patients with HCV genotype 1 with RVR and EVR using Peg–IFN–alpha plus RBV.3,4 The faster the virus becomes clearance during therapy, the better the chance of achieving SVR. Patients with RVR in PBMC with an improved Th1 network can achieve SVR, while those with viral eradication in plasma only without a restored Th1 network have been indicated to relapse.5 Accumulating evidences have shown that an EVR during treatment is determined by serum levels of HCV RNA at week 12 week during therapy.
Recent advances in our understanding of the innate immune responses have indicated that activation of the innate immune response is crucial for subsequent adaptive immune responses, which play key roles in protection against viral infections.6 Persistent HCV infection results from inefficient innate and adaptive immunity.7 Innate immune response regulates adaptive immune responses via direct interactions and by means of the exchange of signals between immune cells.8,9 HCV disturb activation of innate immune responses. The nonstructural proteins of HCV, particularly NS3–4A, have been found to interfere with type I IFN induction pathways. IFNs show the first line of defense against viruses and act directly on viral replication and indirectly via the activation of immune responses.10 The HCV clearance may lead to restoration of innate and adaptive immune responses.11–13 However, there are many problems awaiting solution to induce persistent viral–suppression linked to restoration of innate immune responses resulting in a SVR.
The findings of our previous study showed that cyclic and periodic IFN treatment (CPIT) consisting of induction treatment with natural (n)–IFN–beta and subsequent maintenance treatment with n–IFN–alpha induced restoration of innate immune responses, as showed by the significant decrease of CXCL–10 CXCL–8 and CCL–4, and the significant increase of IL–12 and IL–15 (Figure 1–5); however, insufficient improvements were observed in adaptive immune responses, as showed by the decrease of IFN–gamma and TNF–alpha in CHC patients during novel combination treatment (NCT) consisting of CPIT and subsequent Peg–IFN–alpha/RBV.14,15 Therefore, more effective NCT may be needed in order to improve the rate of SVR in difficult–to–treat CHC patients. The findings of our study support the concept that viral clearance early in the course of therapy is linked to the restoration of innate and adaptive immune responses. It suggests that agents providing viral–suppression leading to RVR and EVR including n–IFN–beta, IFN–lambda, DAAs and new agents may be preferable for an initial early induction therapy. Initial viral clearance induced by CPIT in combination with these agents may lead to the restoration of innate and adaptive immune responses, resulting in a SVR in difficult–to–treat CHC patients. The restoration of innate immune responses may be a novel therapeutic strategy for chronic HCV infection.16
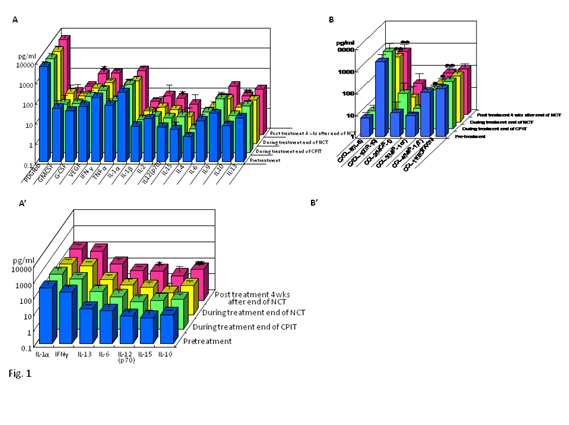
Figure 1 Effect of NCT on serum cytokines (A)(A’) and chemokines (B)(B’) in chronic hepatitis C patients with high viral load, serotype 1 (genotype 1b) and wild or intermediate type of ISDR.
NCT: novel combination treatment.
Significant difference: * p<0.05, ** p<0.1.
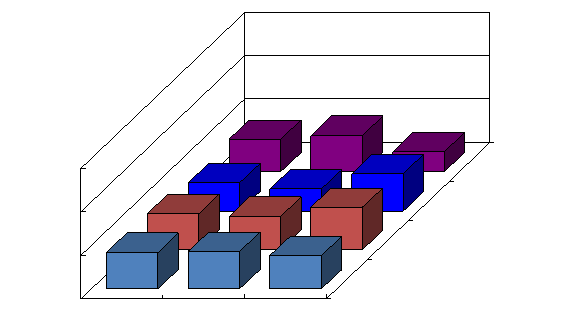
Figure 2 Effect of NCT on serum CXCL–8 in chronic hepatitis C patients with high viral load, serotype 1 (genotype 1b) and wild or intermediate type of ISDR.
NCT: novel combination treatment.
Significant difference: * p<0.05, ** p<0.1.
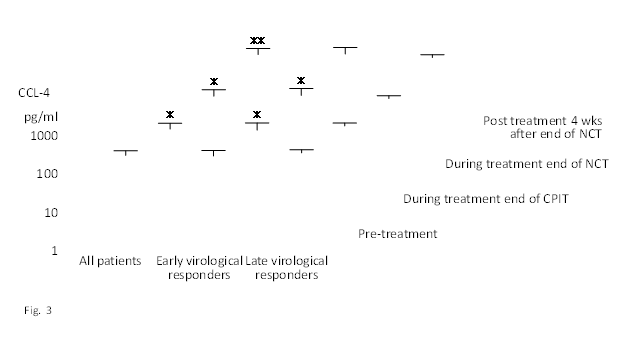
Figure 3 Effect of NCT on serum CCL–4 in chronic hepatitis C patients with high viral load, serotype 1 (genotype 1b) and wild or intermediate type of ISDR.
NCT: novel combination treatment.
Significant difference: * p<0.05, ** p<0.1.
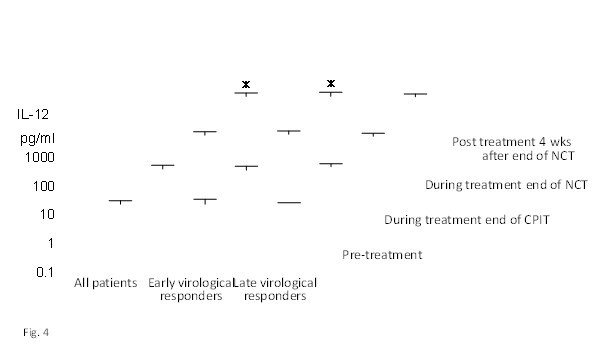
Figure 4 Effect of NCT on serum IL–12 in chronic hepatitis C patients with high viral load, serotype 1 (genotype 1b) and wild or intermediate type of ISDR.
NCT: novel combination treatment.
Significant difference: * p<0.05, ** p<0.1.

Figure 5 Effect of NCT on serum IL–15 in chronic hepatitis C patients with high viral load, serotype 1 (genotype 1b) and wild or intermediate type of ISDR.
NCT: novel combination treatment.
Significant difference: * p<0.05, ** p<0.1.
In addition to the restoration of innate immune responses due to viral–suppression with CPIT during the initial early course of therapy, persistent virologic clearance with triple therapy consisting of a protease inhibitor (Simeprevir) plus Peg–IFN–alpha/RBV were more likely to result in a higher RVR, EVR, end–treatment virologic response (ETVR), and SVR. On the basis of these findings, we conducted to treat a CHC patient with genotype 1b, a high viral load, a prior null response to IFN treatments and chronic active hepatitis with advanced fibrosis using induction therapy with n–IFN–beta followed by triple therapy with Simeprevir plus Peg–IFN–alpha–2b/RBV, which resulted in SVR and sustained biochemical response (SBR).
Our patient
We treated a CHC patient.48 years old, male, serotype 1 (genotype 1b), HCV RNA levels of 6.7 Log IU/ml, Grade 3 and Stage 3–4, who was obese (height 171.0 cm, body weight 82.7 kg, BMI 28.3 kg/m2) and receiving treatment for hypertension and insomnia. His serum alanine amino transferase (ALT) level was 68 IU/l, serum total bilirubin level was 1.2 mg/dl (direct bilirubin 0.6 mg/dl), serum albumin level was 3.3 g/dl, platelet count was 10.3 x 104 /micro–l, prothrombin time was 69.7 %, and he was negative for the HBs–antigen, HBc–antibody and antinuclear antibody. A liver biopsy sample, which was collected within 1 week of the initiation of treatment, revealed chronic hepatitis (active stage) with advanced fibrosis (Grade 3 Stage 3–4).
Induction therapy with natural Interferon–beta followed by triple therapy with Simeprevir plus Peg–IFN–alpha–2b/RBV Therapy
Simeprevir was administered at the dose of 100 mg (one capsule) once per day. Peg–IFN–alpha–2b was used at the weight–based dose of 1.5 micro–g / kg/ week. RBV dose was 200–1000 mg/day based on body weight. The patient was treated with a cyclic and periodic interferon treatment (CPIT) for 26 weeks as induction therapy and subsequent triple therapy with Simeprevir (Sovriad, Janssen, Titusville, NJ, USA, 100 mg/day per os, daily) plus Peg–IFN–alpha–2b (Pegintron, MSD, Whitehouse Station, NJ, USA, 100–120 micro–g/day, once a week, percutaneous inj) and RBV (Rebetol, MSD, Whitehouse Station, NJ, USA, 200–1000 mg/day, per os, daily) for 12 weeks, and subsequently with Peg–IFN–alpha–2b and RBV for 18 weeks (a total of 56 weeks). The patient was treated with 7 cycles of CPIT (26 weeks). One cycle of CPIT consisted of induction treatment with n– IFN–beta(Feron, Toray Co. Ltd., Chiba, Japan)at 6 MU/day, intravenously by a drip infusion in 100 ml of saline solution, daily for 2 weeks, and this was followed by maintenance treatment with n–IFN–alpha (Sumiferon, Sumitomo Co. Ltd., Osaka, Japan) at 3–6 MU/day, self–subcutaneously inj., every two days for 2 weeks. Co–administration of Simeprevir with substrates that are moderate or strong inducers or inhibitors of cytochrome P450 3A (CYP3A) was not used. The patient with chronic active hepatitis C with advanced fibrosis was treated under careful monitoring and informed consent. The patient provided written informed consent.
The patient was checked with clinical, biochemical, and virologic findings before and every 1 to 4 weeks during the treatment, and more than 12 weeks after cessation of the therapy. Serum levels of HCV RNA were determined using the quantitative COBAS AMPLICOR HCV MONITOR test, ver. 2.0 (Roche Diagnostic Systems, Tokyo, Japan; sensitivity < 50 IU/ml). The goal of the therapy is to eradicate HCV infection in order to prevent liver cirrhosis, de compensation of cirrhosis, hepatocellular–carcinoma and death. The endpoint of therapy is persistent virologic clearance of HCV RNA 12 weeks after cessation of the therapy (i.e., a SVR).
Assessments of safety
Adverse effects (AEs) were checked every 1–4 week during the therapy and after cessation of the therapy. Laboratory tests, vital sign, a fatigue symptom, a flu–like symptom and electrocardiogram assessments, and physical examinations were carried out at screening and thereafter at regular intervals during the treatment. The RBV dose was reduced from 1000 mg to 200 mg per day to manage AEs or laboratory abnormalities.
Measures to improve treatment adherence
Full adherence to all drugs is associated with high SVR rates. On the contrary, suboptimal exposure to therapy is associated with virological breakthrough or post–treatment relapse and the emergence of resistance–associated variants, especially during the early phase of treatment. Before starting antiviral therapy, the patient was instructed about the schedule and the side effects to be expected during treatment. The patient was instructed about the preventive and therapeutic measures to ameliorate these side effects. Regular follow–up visits were scheduled so that treatment progress and management of side effects were discussed.
The patient was initially administered a low dose of Peg–IFN–alpha–2b of 100 micro–g/day, once a week for 2 weeks; however, this dose was increased to a weight–balanced dose of 120 micro–g/day due to the development of mild AEs. RBV was initiated at a weight–balanced dose of 1000 mg/day and was administered for 4 weeks. This dose was reduced to 800 mg/day for 2 weeks due to decrease in hemoglobin levels from 14.3 g/dl before treatment to 11.0 g/dl and was again reduced to 200 mg/day due to a further decrease in hemoglobin levels from 11.2 g/dl to 9.7 g/dl. RBV at a daily dose of 200 mg /day was administered for 26 weeks to manage anemia. After cessation of the treatment, hemoglobin levels recovered from 8.4 g/dl to 14.1 g/dl (Figure 6).

Figure 6 Serial determinations of serum hemoglobin levels (Hb), platelet count (Plt) and total bilirubin levels (T.B.) in a patient with chronic hepatitis C [a 48 year old, male, BMI 28.3 kg/m2, serotype 1 (genotype 1b), HCV RNA level of 6.7 LogIU/ml, chronic active hepatitis with advanced fibrosis (Grade 3, Stage 3–4)] who was treated with CPIT for 26 weeks followed by Simeprevir plus Peg–IFNalpha–2b/RBV for 12 weeks, and subsequently with PegIFN–alpha–2b/RBV for 18 weeks. CPIT: a cyclic and periodic IFN treatment consisting of an induction treatment with natural IFN–beta followed by a maintenance treatment with natural IFN–alpha.
Platelet levels were reduced from 10.3 x104 /micro–l before treatment to 5.1 x104 /micro–l and were managed at more than 5.0 x104 /micro–l during the treatment. Platelet levels improved from 5.6 x104 /micro–l at the end of therapy to 9.5 x104 /micro–l 4 weeks after cessation of the treatment and increased to 9.6 x104 /micro–l 12 weeks after cessation of the therapy. Anemia and thrombocytopenia were managed by dose reduction in the RBV dose from 1000 mg/day to 800 mg/day, and subsequently with a daily RBV dose of 200 mg/day without virologic breakthrough to manage AEs or laboratory abnormalities. No reduction of dose of Peg–IFN–alpha–2b was needed to manage anemia and thrombocytopenia. Anemia and thrombocytopenia were managed without discontinuation of the treatment. The treatment was successfully completed (Figure 6).
Serum levels of HCV RNA levels decreased after 4 weeks of CPIT. CPIT markedly decreased serum HCV RNA levels from 6.7 Log IU/ml before treatment to less than 1.2 Log IU/ml 12 weeks after CPIT with complete EVR (cEVR) and ETVR. CPIT was tolerated well and only mild AEs developed. The subsequent Simeprevir plus Peg–IFN–alpha–2b/RBV treatment caused persistent virologic clearance without virologic breakthrough and relapse resulting in an ETVR and a sustained virologic response 12 weeks after cessation of the treatment (SVR 12) (Figure 7).
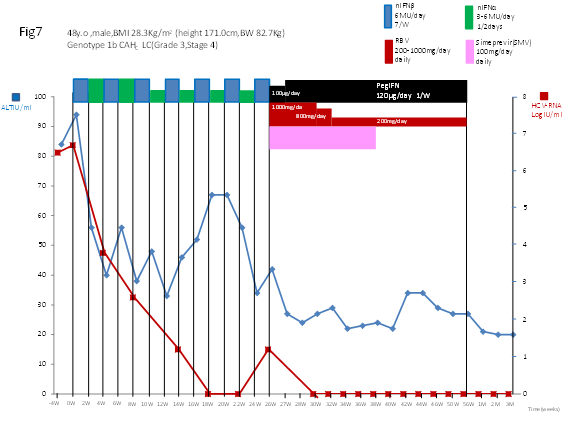
Figure 7 Serial determinations of serum levels of HCV.RNA and alanine amino transferase (ALT) in a patient with chronic hepatitis C [a 48 year old, male, BMI 28.3 kg/m2, serotype 1 (genotype 1b), HCV RNA level of 6.7 LogIU/ml, chronic active hepatitis with advanced fibrosis (Grade 3, Stage 3–4)] who was treated with CPIT for 26 weeks, followed by Simeprevir plus Peg–IFN–alpha–2b/RBV for 12 weeks and subsequently with Peg–IFN–alpha–2b/RBV for 18 weeks.
CPIT: A cyclic and periodic IFN treatment consisting of an induction treatment with natural IFN–beta followed by a maintenance treatment with natural IFN–alpha.
Serum levels of ALT decreased within 3 weeks of the initiation of CPIT and serum ALT levels were reduced from 94 IU/l before treatment to 37 IU/l, which was within the normal range. Serum ALT levels were only slightly elevated during the CPIT. After the initiation of triple therapy, serum levels fell into the normal range for ALT and subsequently remained normal; serum levels of ALT remained normal during the treatment and 12 weeks after cessation of the treatment (SBR) (Figure 7).
A slight, transient increase in serum bilirubin concentration was observed during the treatment with Simeprevir plus PegIFN–alpha–2b/RBV. These transient elevations in bilirubin were not associated with concomitant elevations in serum amino transferases, alkaline phosphatase, or hepatic de compensation during the Simeprevir dosing intervals. Birilubin levels were slightly elevated for the entire period of triple therapy and fell into the normal range after the cessation of triple therapy (Figure 6). Serum levels of albumin slightly decreased during the treatment and improved from 3.3 g/dl before treatment to 3.7 g/dl 12 weeks after cessation of the treatment. The prothrombin time improved from 69.7% before treatment to 85.7%, which was in the normal range, 8 weeks after the initiation of CPIT. The prothrombin time remained at more than 70 % during the treatment and subsequently remained normal 48 weeks after initiation and after cessation of the treatment (Figure 8). Improvement in the prothrombin time and total bilirubin levels were observed 12 weeks after cessation of the treatment. The principal AEs of the IFN treatment were fatigue, arthralgia, nausea, anorexia and insomnia.
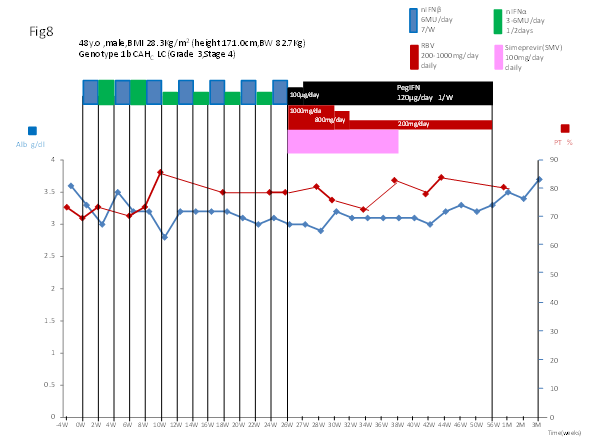
Figure 8 Serial determinations of serum levels of albumin (Alb) and the prothrombin time (PT) in a patient with chronic hepatitis C [a 48 year old, male, BMI 28.3 kg/m2, serotype 1 (genotype 1b), HCV.RNA level of 6.7 LogIU/ml, chronic active hepatitis with advanced fibrosis (Grade 3, Stage 3~4)] who was treated with CPIT for 26 weeks followed by Simeprevir plus Peg–IFN–alpha–2b/RBV for 12 weeks, and subsequently with Peg–IFN–alpha–2b/RBV for 18 weeks.
CPIT: a cyclic and periodic IFN treatment consisting of an induction treatment with natural IFN–beta followed by a maintenance treatment with natural IFN–alpha.
These AEs are commonly associated with IFN treatment. The severity of these AEs was slightly increased by the administration of Simeprevir plus Peg–IFN–alpha–2b/RBV. A decrease in the dose of RBV was needed to manage AEs. Furthermore, the triple therapy induced a slightly depressive reaction that was controlled well with an anti–depressant. The patient developed tachycardia in week 8 when n–IFN–alpha was administered. Then, n–IFN–alpha was reduced to 3 MU/day due to tachycardia and this was controlled well with a beta–blocker and improved after cessation of the treatment with n–IFN–alpha. AEs were less during treatment with n–IFN–beta than treatment with n–IFN–alpha or triple therapy. The severity of these AEs decreased when the dose of RBV was reduced and the administration of Simeprevir was completed. Anorexia, fatigue, anemia, thrombocytopenia and the prothrombin time improved 4 weeks after cessation of the treatment. The severity of these AEs was mild. The patient successfully completed the induction therapy with n–IFN–beta followed by Simeprevir plus Peg–IFN–alpha–2b/RBV.
We herein described for the first time the successful treatment of a difficult–to–treat CHC patient with genotype 1b, a high viral load, a null response to previous IFN treatments and advanced hepatic fibrosis with induction therapy with n–IFN–beta, which reduced serum levels of HCV RNA, followed by Simeprevir plus Peg–IFN–alpha–2b/RBV and achieved SVR 12 and SBR. The treatment success is particularly important for patients with advanced hepatic fibrosis, the mortality of whom is high without successful treatment. We conducted to treat a CHC patient with genotype 1b, a high viral load, a prior null response to IFN treatments, and chronic active hepatitis with advanced fibrosis using induction therapy with nIFN–beta followed by triple therapy with Simeprevir plus PegIFN–alpha–2b/RBV, which resulted in SVR without virologic breakthrough and relapse, SBR and improved prothrombin time. This study revealed that the induction therapy followed by triple therapy was tolerated well and enhanced RVR, cEVR, ETVR, and SVR in the difficult–to–treat CHC patient, who only developed mild AEs with the exception of bilirubin, for which a slight and transient increase in serum bilirubin concentration was observed during the treatment with Simeprevir plus PegIFN–alpha–2b/RBV. In vitro studies have revealed that Simeprevir is an inhibitor of the bilirubin transporters (OATP1B1 and MRP2), suggesting a likely mechanism for the observations. Simeprevir is a potent inhibitor of OATP1B1, which is primarily responsible for transporting un conjugated (indirect) bilirubin, than MPR2, primarily a conjugated (direct) bilrubin transporter.17
This higher virologic response rate highlights the benefit of induction therapy followed by triple therapy in difficult–to–treat CHC patients. The results of this study support the concept that viral clearance early in the course of therapy is effective for difficult–to treat CHC patients. In this study, the induction therapy was followed by a prompt and marked decrease in serum HCV RNA levels in the well–documented CHC patient with genotype 1b, a high viral load, advanced hepatic fibrosis and null response to previous IFN treatments. The decrease observed in HCV RNA levels occurred by the initiation of CPIT, and persistent viral clearance with subsequent triple therapy showed that the induction therapy followed by triple therapy was effective therapy for difficult–to–treat CHC patients. Our results suggest that agents providing viral suppression leading to RVR and EVR including n–IFN–beta, DAAs and newly developed agents are preferable for the initial early induction therapy. IFNs show the first line of defense against viruses and act directly on viral replication and indirectly via the activation of immune responses.10 IFN–beta was previously shown to have different signaling and biological activities from those of IFN–alpha, and achieved a higher rate of viral clearance than IFN–alpha.18–23 The incidence of AEs such as depression, alopecia, general fatigue, and hematological changes were lower during therapies with n–IFN–beta than those with IFN–alpha.24–26 In constrict to the actions of IFN–alpha, IFN–beta and IFN–lambda signaling in the liver did not become refractory during stimulation of the IFN signaling transduction pathway. The proved efficacy of IFN–beta and IFN–lambda may be crucial for the treatment of patients who do not respond to Peg–IFN–alpha via a pre–activated endogenous IFN system.27 Bolen et al.28 reported that Type I and III IFNs differed greatly in the levels of IFN stimulated gene (ISG) induction with a clearly detectable hierarchy (IFN–beta > IFN–alpha > IFN–rhamda–3 > IFN–rhamda–1 > IFN–rhamda–2). In the case of IFN–alpha, the induction of an antiviral state appeared to be transient, with ISG expression starting to decline after 6 hours post–stimulation due to a negative feedback mechanism. On the contrary, IFN–beta and all three IFN–rhamdas induced long–lasting gene expression.28 A transcriptome analysis from human hepatoma Huh7 cells or primary human hepatocytes (PHHs) stimulated with similar concentrations of IFN–alpha, –beta, rhamda–1, rhamda–2, or rhamda–3 showed a hierarchy of gene expression, with IFN–beta being identified as the most biologically active cytokine, followed by IFN–alpha and IFN–rhamdas. Both IFN–beta and IFN–rhamda triggered a sustained ISG response; on the other hand, IFN–alpha produced a kinetic profile of genes that peaked early in the therapy and then rapidly decreased (Figure 9). No significant difference was observed in STAT1 tyrosine phosphporylation levels between IFN–beta and IFN–alpha, where as serine phosphporylation at position 727 was stimulated more strongly by IFN–beta than by IFN–alpha.29 The finding that IFN–beta invariably possessed the highest activity among Type I and Type III IFNs suggested that IFN–beta and IFN–rhamda–3 may have superior clinical activity.
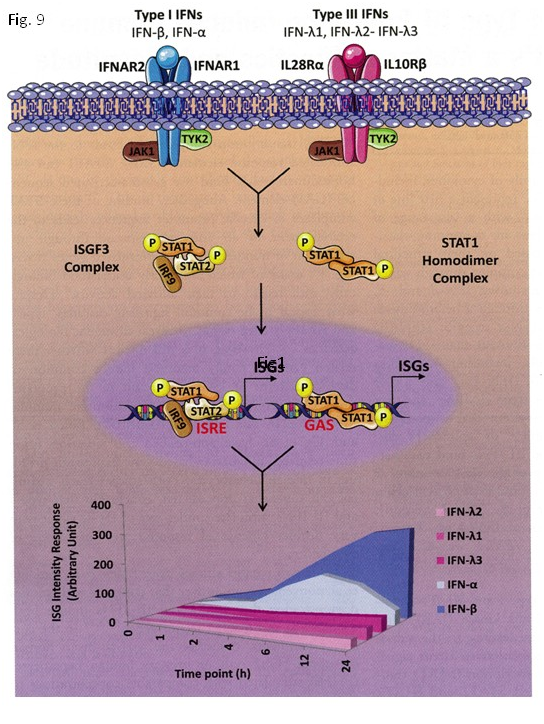
Figure 9 Type I and III IFN–signaling pathways lead to different kinetics and strengths of stimulation of the ISG response. Both type I and III IFNs induced STAT phosphorylation through the JAK–TYK kinases associated with the respective receptor subunits. Receptor engagement resulted in the activation of STA1 and STAT2, which complexed with IRF9 to form the transcription factor, ISGF3. STAT1 also homodimerized after being phosphorylated. These complexes induced the expression of genes with ISRE or GAS in their promoters, leading to different kinetics and magnitudes of the ISG stimulation. The graph below indicates the relative abundance, kinetics, and magnitude of the resulting ISG response to type I and III IFN.29
Mondelli et al.30 found that RBV played a role as a modulator of innate immune responses. The up–regulation of ISG was greater in patients treated with Peg–IFN–alpha/RBV than in those treated with IFN–alpha alone, suggesting a role for RBV as a modulator of innate immune responses. These findings show numerous potential clinical applications of RBV as an immuno–modulator (the effect of RBV on innate and adaptive immune responses).30 (Figure 10). Werner et al.31 addressed, for the first time, the hypothesis that NK cell function may be influenced by exposure to RBV in vitro and in vivo before Peg–IFN–alpha/RBV therapies. They reported interesting, and up to the present unrecognized effects of RBV, which reduced phosphorylated signal transducer and activator of transcription (pSTAT) 4 levels in NK cells, but had no effect on STAT1 phosphorylation in vitro and in vivo, resulting in the increased inducibility of pSTAT4 and production of pSTAT4 independent IFN–gamma in response to IFN–alpha.31

Figure 10 Effects of RBV on innate and adaptive immune responses. RBV lowered the baseline expression of ISG and resets IFN responsiveness in the liver, making the liver more permissive to the action of exogenous IFN. This drug also improved the inducibility of pSTAT4 in NK cells during conventional IFN–alpha/RBV combination treatment in increased proportions of IFN–gamma–producing NK cells. The effects of RBV on adaptive immunity mainly consisted of switching CD4 T–cell responses to a Th1 profile favored by the RBV–mediated suppression of IL–10 secretion by Tregs, which resulted in the improved efficiency of CD4 T–cell effectors secreting IFN–gamma.30
Therefore, IFN–beta and RBV may effectively restore innate immune response in impaired innate and adaptive immune responses in difficult–to–treat CHC patients. In previous studies, dose decreases or discontinuations of Peg–IFN–alpha, which were often needed to manage AEs, have been associated with a decrease in treatment efficacy. No serious AEs were found in induction therapy with n–IFN–beta followed by triple therapy, and good tolerance was confirmed.
Persistent HCV clearance was continued in our patient with a decrease in HCV RNA due to induction therapy and subsequent triple therapy, even when RBV was administered at a low dose. These results suggest that induction therapy associated with decrease in HCV RNA levels before the initiation of triple therapy was suggested to be more effective than triple therapy alone to treat difficult–to–treat CHC patients. The results of this preliminary study indicate the need for a prospective assessment of the possible role of viral–suppression linked to restoration of innate immune responses with induction therapy followed by triple therapy in difficult–to–treat CHC patients.
Viral–suppression linked to restoration of innate immune responses with induction therapy with n–IFN–beta and subsequent Simeprevir plus Peg–IFN–alpha–2b/RBV was tolerated well without discontinuation, and overcame viral breakthrough and relapse, leading to an early virologic response, SVR 12 and SBR in a difficult–to–treat CHC patient with a high viral load, genotype 1b, a null response to prior IFN treatments and chronic active hepatitis with advanced fibrosis. It also effectively eradicated HCV infection and caused mild adverse effects in a difficult–to–treat CHC patient, even when RBV was administered at a low dose.
None.
None.

©2015 Kishida, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.