MOJ
eISSN: 2373-4442


Research Article Volume 6 Issue 2
Department of Pediatrics, Research and Training Hospital of Sakarya University,Turkey
Correspondence: Mohsen Khalili, Department of Immunology, University of Medical Sciences, Iran,, Tel 989178000464
Received: January 01, 1971 | Published: March 13, 2018
Citation: Khalili M. Serum levels of interleukin (IL)-33 in patients with ischemic heart disease. MOJ Immunol. 2018;6(2):29-32. DOI: 10.15406/moji.2018.06.00188
Objective: It has been reported that cytokines play a crucial role in pathogenesis of different diseases such as cardiovascular diseases. The aim of this study was to evaluated the serum level of IL-33 in patients with ischemic heart disease (IHD) and also clarify its association with traditional risk factors of the disease.
Methods: A total of 300 patients with IHD as having acute myocardial infarction (AMI; n=100), unstable angina (UA; n=100) or stable angina (SA; n=100) and 100 sex- and age- matched healthy subjects as control group (n=100) were enrolled to this cross-sectional, case controlled study. Serum samples were collected from all participants and tested for IL-33 by use of ELISA method.
Results: The mean serum concentration of IL-33 in AMI (103.33±19.29 Pg/ml), SA (157.60±33.43 Pg/ml) and UA (122.21 ± 22.26 Pg/ml) was significantly higher than that observed in healthy control (61.85±7.67 Pg/ml). There was no significant difference between patient with or without certain traditional risk factor including hypertensionve patients, dyslipidemic patients, smokinger, obesity e patients and diabetesic patients in the mean serum level of IL-33.
Conclusion: These results showed that the higher serum concentration of IL-33 was associated with IHD. The presence or absence of certain traditional risk factors of IHD did not influence the serum level of cytokines.
Keywords: ischemic heart disease, acute myocardial infarction, unstable angina, stable angina, interleukin (IL)-33
Different studies demonstrated that immunopathological and inflammatory mechanisms play a crucial role in the development of IHD. Accumulation of different immune cells including B cells, T cells, monocyte/ macrophages and PMNs has been showed in atherosclerotic lesions.1,2 Immune responses, especially T- helper- dependent responses play an important role in the pathogenesis of cardiovascular diseases.3 Helper T cells are arguably the most important cells in adaptive immunity, as they are required for almost all adaptive immune responses.4 T helper cells can differentiate to Th1 and Th2 subsets upon antigenic stimulation. Each subsets release distinct cytokine profiles. Different studies have showed that imbalance between Th1 and Th2 cells are associated with cardiovascular diseases.5 The mechanisms responsible for Th1 and Th2 imbalance in IHD are not well understood. However, studies have demonstrated the up-regulation of Th1-associated inflammatory response and down-regulation of anti-inflammatory responses of Th2-related cytokines is associated with cardiovascular diseases.3–8 Interleukin 33 (IL-33) is a cytokineprotein that in humans is encoded by the IL33 gene. IL33 is a ligand for ST2 (IL1RL1), an IL-1 family receptor that is highly expressed on Th2 cells, mast cells and group 2 innate lymphocytes.9 IL-33 is expressed by a wide variety of cell types, including fibroblasts, mast cells, dendritic cells, macrophages, osteoblasts, endothelial cells, and epithelial cells.10 Interleukin 33 is a member of the IL-1 family that potently drives production of T helper-2 (Th2) associated cytokines (e.g., IL-4).11 These observations suggest that IL-33 may have a modulatory role in IHD. Although, there are some studies on the serum levels of cytokines, such as IL-1, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, IL-13, IL-17, IL-18 and granulocyte–macrophage colony-stimulating factor (GM-CSF) in patients with IHD,12–15 however, there is no data on IL-33 and the relationship of cytokine with traditional risk factors of IHD is not also clarified. Therefore, this study conducted for the first time to evaluate the serum level of IL-33 in patients with IHD and also to clarify its association with traditional risk factors of the disease.
Patients
A total of 300 patients (aged 40-60) with IHD who admitted to Afzalipour hospital of Kerman were enrolled in this study. Patients were classified into 3 groups according to criteria as, AMI (n=100), stable angina (n=100) or unstable angina (n=100). The diagnosis of AMI was established according to the presence of two of the these criteria: i-prolonged chest pain compatible with AMI, ii- raising of cardiac enzymes, iii- typical ECG changes. Stable angina (SA) was defined as effort angina of at least 6 months period without any change in symptom frequency or severity and angiographically documented obstructive coronary artery diseases (CAD). Unstable angina was defined according to the BraunWald’s classification and all patients had chest pain at rest with definite ischemic ECG changes such as ST-segment changes and/or T-wave inversion. Patients with UA were in class IIIB according to BraunWald’s classification.12 Control group was comprised of 100 sex- and age-matched subjects with similar geographic and socioeconomic background without IHD. The healthy control group was recruited among blood donors of Kerman Blood Transfusion Center. The control subjects were apparently healthy with a normal blood pressure, normal body temperature, and normal pulse rate and without tachycardia. Exclusion criteria were vascular heart disease, surgery, trauma within the prior month, cardiomyopathy, liver disease, renal failure, malignant diseases, other inflammatory disease (such as septicemia and pneumonia) and oral anticoagulant therapy. 2–4 ml of peripheral blood was collected from the patients and healthy subjects and the serum separated and stored at -20 oC. The study was approved by the Ethics Committee of Kerman University of Medical Sciences. Moreover, patients were recruited if they agreed for blood sampling.
Interleukin assay
The serum concentrations of IL-33 were quantified by sandwich ELISA using commercial kits (R&D Systems, Minneapolis, MN). The serum levels of cytokine were quantitated by using standard samples with known concentration of cytokine expressed as Pg/ml, provided by the manufacturer.
Statistical analysis
All the available data were analyzed by a computer program (SPSS, Chicago, IL, USA). Differences in variables were analyzed using Student -t or ANOVA (for normally distributed data) Mann-Whitney U or Kruskal-Wallis tests (for non-normally distributed data) as appropriate and P values of less than 0.05 were considered significant.
Baseline characteristics of patients
Table 1 lists the baseline characteristics of AMI, SA, UA and healthy control group subjects. There were no significant differences among 4 groups for the age and gender. Besides, no statistically significant differences were observed between both group’s patient with respect to age and the presence of traditional risk factors of the diseases.
Serum levels of IL-33 in IHD and control groups
The mean serum level of IL-33 in AMI, SA, UA and healthy group were Pg/ml, 103.33±19.29 Pg/ml, 157.60±33.43 Pg/ml, 122.21±22.26 Pg/ml and 61.85±7.67 Pg/ml, respectively. Statistical analysis demonstrated that the mean serum concentration of IL-33 in AMI, SA or UA were significantly higher as compared with the healthy control group (Figure 1 & Table 1) Moreover, no significant difference was observed between different patients groups (AMI and SA, AMI and UA, SA and UA). Our statistical analysis also showed that the mean serum level of IL-33 in total IHD patients were significantly higher than those observed in healthy subjects (P< 0.001). No significant difference was observed between men and women regarding the mean serum level of IL-33 in healthy control and IHD groups. (Table 2) No significant differences were observed between patients groups with respect to the age, sex or presence of risk factors. (Table 3) shows the serum levels of IL-33 in patients with IHD according to traditional risk factors. Our results also showed no significant difference between patient with a certain risk factor (including hypertensive patients, dyslipidemic patients, smoker, obese patients and diabetic patients) and patients without a certain traditional risk factor (including non-hypertensive patients, non-dyslipidemic patients, non-diabetic patients, non-obese patient and non-smoker patients) in the mean serum level of IL-33.
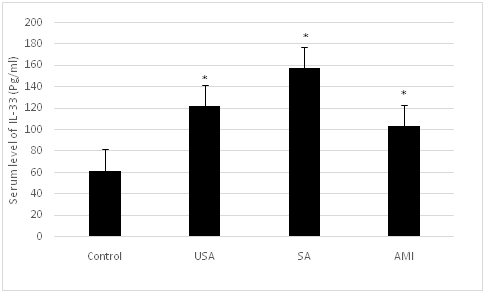
Figure 1Mean serum concentrations of IL-33 in AMI, UA, SA and healthy control groups.
*Statistical analyses by using ANOVA and student t tests showed that the mean serum concentrations of IL-33 in AMI, SA and UA groups were significantly higher than in healthy control group (P<0.001).
UA (n = 100 |
SA (n = 100 |
AMI (n = 100) |
Control (n = 100 |
|
Age, mean±SD |
56.3±9.2 |
57.1±9.7 |
58.8±6.1 |
59.2±10.0 |
Sex (Men/women) |
67/33 |
64/36 |
68/32 |
0 |
Hypertension |
42 |
36 |
44 |
0 |
Diabetes mellitus |
27 |
15 |
22 |
0 |
Current smoking |
18 |
20 |
27 |
0 |
Obesity |
28 |
33 |
34 |
0 |
Dyslipidemi |
19 |
21 |
38 |
0 |
Table 1 Baseline characteristics of study patients
Group |
Sex |
No |
IL-33 levels* (mean±SD) |
P value |
UA |
Male |
67 |
126.48±27.18 |
0.16 |
Female |
33 |
113.54±24.80 |
||
Total |
100 |
122.21±22.26 |
||
SA |
Male |
64 |
164.48±39.76 |
0.32 |
Female |
36 |
145.37±34.45 |
||
Total |
100 |
157.60±33.43 |
||
AMI |
Male |
69 |
108.83±24.67 |
0.24 |
Female |
31 |
91.12±21.3 |
||
Total |
100 |
103.33±19.29 |
||
Control |
Male |
68 |
63.66±11.7 |
0.11 |
Female |
32 |
58.03±9.08 |
||
Total |
100 |
58.03±9.08 |
Table 2 Serum levels of IL-33 in IHD and healthy groups according to gender
*The serum levels of cytokine expressed as Pg/ml
Risk factor (RF) |
RF status |
No. |
IL-33 levels* (mean±S) |
P value |
Hypertension |
Positive |
122 |
121.48±21.67 |
0.21 |
Negative |
178 |
131.98±24.66 |
||
Diabetes mellitus |
Positive |
64 |
117.26±20.34 |
0.16 |
Negative |
246 |
125.24±23.71 |
||
Current smoking |
Positive |
65 |
98.58±18.23 |
0.46 |
Negative |
235 |
135.77±26.06 |
||
Obesity |
Positive |
95 |
101.45±21.8 |
0.43 |
Negative |
205 |
139.88±24.16 |
||
Dyslipidemia |
Positive |
78 |
124.81±20.47 |
0.11 |
Negative |
222 |
128.73±25.39 |
||
Without RF (healthy group) |
100 |
61.85±7.67 |
||
Table 3 Serum levels of IL-27 in patients with IHD according to traditional risk factors
*The serum levels of cytokine expressed as Pg/ml
Our data showed that the mean serum concentration of IL-33 in AMI, SA or UA were significantly higher as compared with the healthy control group. IL-33 is a recently identified member of IL-1 family which is expressed on epithelial cells, smooth muscle cells, keratinocytes, fibroblasts, endothelial cells, dendritic cells and activated macrophages.16–18 The receptor for IL-33 is a heterodimer consisting of ST2 and IL-1 receptor accessory protein. The ST2 encode at least 3 isoform of ST2 proteins by alternative splicing: ST2L, a trans-membrane receptor, secreted soluble ST2 (sST2) form which can act as a decoy receptor for IL-33; and ST2V which is a variant form present mainly in the gut of humans.19–21
The increased serum level of IL-33 in patients group may be attributed to the myocardial damages. IL-33 can act as alarming and studies suggested that IL-33 is specially released during necrotic cell death, which is thought to be associated with tissue damage during infection and trauma.22,23 Studies demonstrated that IL-33 is a multifunctional immunomodulatory cytokine that acts both pro- and anti-inflammatory depending on the disease.9–24 Our data showed that SA patients had a higher mean concentration of IL-33 than UA patients. Moreover, UA patient also had higher concentration of this cytokine in comparison to AMI patients that are in acute situation. Previous studies suggested beneficial effect of IL-33 in cardiovascular diseases such as atherosclerosis, obesity and type 2 diabetes, cardiac fibrosis and hypertrophy.19 Studies also showed that ST2L selectively expressed on Th2, but not Th1 or regulatory T (Treg) cells and IL-33 stimulation of Th2 cells is associated with the production of Th2 cytokines IL-4, IL-5 and IL-13.11–25 It has been reported that Th1/Th2 imbalance may be involved in the pathogenesis of IHD so that the down regulation of Th2-type cytokines such as IL-4 and IL-10 and up-regulation of Th1-type cytokines such as IFN-ɣ, IL-12 and IL-18 have been reported in IHD.3–8 It seems that higher concentration of IL-33 in SA patients in comparison of UA and AMI patients play a modulatory and anti-inflammatory role and cause better situation in SA patients. In our study, we didn’t measure the level concentration of sST2 as a decoy receptor of IL-33. Previous study showed that sST2 level were significantly increased in cardiovascular diseases,19,22 so level measurement of sST2 can help us to better understanding the role of IL-33 in IHD. The results of the present study also showed no significant difference between patients with or without certain risk factor in the mean serum level of IL-33. These finding suggested that IL-33 may not dependently (in the context of conventional risk factors) or independently (in the absence of conventional risk factors) contribute to IHD events.
In conclusion, our results showed that the higher serum concentration of IL-33 were associated with IHD. Moreover, the presence or absence of certain traditional risk factors of IHD did not influence the serum level of cytokines.
This research was supported by a grant [NO: 94-169] from the Physiology Research Center, Kerman University of Medical Sciences, Kerman, Iran.
None.

©2018 Khalili. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.