MOJ
eISSN: 2373-4442


Mini Review Volume 4 Issue 3
Department of Clinical Sciences, University of Sharjah, UAE
Correspondence: Maha Mosheer Guimei, College of Medicine University of Sharjah, UAE,, Tel 00971-5-02241727, Fax 00971-6-5585879
Received: January 01, 1971 | Published: November 11, 2016
Citation: Guimei MM, Barqawi HJ, Dash NR, Maghazachi AA (2016) Sepsis- The Dilemma Continues. MOJ Immunol 4(3): 00125. DOI: 10.15406/moji.2016.04.00125
The reviewed evidence here suggests that sepsis is a heterogeneous disorder involving several body systems, organs, tissues, proteins and molecules. Alerted microcirculation resulting in tissue metabolism remains the ultimate outcome of this interplay. The cascade initiation resulting in endothelial injury, complement activation, coagulopathy, and microcirculatory dysfunction is conclusively the interaction between microbes and human host. Better understanding of these interplays and quantitative description of the underlying pathogenesis mechanisms will help us answer several unanswered questions in sepsis.
Keywords: sepsis, anti-TNF, interleukin-1, dilemma continues, PRRs, inflammatory, iNOS, immune response, endogenous factors, TF
SIRS, systemic inflammatory response syndrome; immunosuppression; PAMPs, Pathogen-Associated Molecular Patterns; TNF, tumour necrosis factor; TLRs, toll-like receptors; DIC, disseminated intravascular coagulopathy; TF: tissue factor; iNOS, inducible nitric oxide synthase; APC, activated protein C
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection.1 Sepsis syndrome represents the body’s host response to infection. Classically, sepsis has been defined and classified as systemic inflammatory response syndrome (SIRS), sepsis and septic shock based on diagnostic criteria derived from the systemic response to inflammation (Figure 1). However, recently the term ‘severe sepsis’ has been dropped from the definitions to better identify, manage, report and improve outcomes.1 The prevalence of sepsis is on the rise. Factors could be many; such as an ageing population, immunosuppression, evolving multidrug resistant pathogens, more people in the ICUs, as well as an increase in the number of low-birth-weight new-borns who are particularly at a high risk.2
Sepsis is a dysregulated host response to a suspected infective agent that is amplified by endogenous factors.3,4 To date, there is no single mediator or pathway that drives the pathogenesis of sepsis but rather a multifaceted response that involves interplay between various body systems; an inflammatory/ immune response, cardiovascular, neuronal, hormonal, metabolic and coagulation.3–5 This interplay will ultimately contribute to either the progression to septic shock and multiorgan dysfunction or the complete walling off of the infection thus limiting its effects on the rest of the body organs. Understanding such interplay is of fundamental importance in order to guide new diagnostic and management strategies.
In the past, sepsis was mostly associated with gram-negative bacterial infections. This trend has changed over recent years with more sepsis being triggered by commensal microbes and gram-positive bacteria like staphylococci and enterococci.6 The prevalence of fungal sepsis has also increased over the past decade, but remains lower than bacterial sepsis.7,8
Sepsis caused by gram-negative bacteria is thought to be largely due to the host's response to the lipid A component of lipopolysaccharide present on the cell wall, whereas, sepsis caused by gram-positive bacteria can result from an immunological response to cell wall lipotechoic acid. Besides, several microbial factors can initiate septic inflammatory cascade and behave as superantigens. An invading pathogen is recognized by its pathogen-associated molecular patterns (PAMPs). Examples of PAMPs include lipopolysaccharides and flagelin in gram-negative bacteria, muramyl dipeptide in the peptidoglycan of the gram-positive bacteria cell wall, and CpG bacterial DNA. These PAMPs are recognized by the innate immune system’s pattern recognition receptors (PRRs), which can be membrane-bound or cytosolic.10 There are four families of PRRs: the toll-like receptors, the C-type lectin receptors, the NOD-like receptors and the RIG-I-like receptors. The association of a PAMP and a PRR will invariably cause a series of intracellular signalling cascades leading to activation of transcription factors like nuclear factor-kappa B and activator protein-1. This ultimately leads to overlapping downstream pathways that will up-regulate the expression of pro-inflammatory and anti-inflammatory molecules like tumour necrosis factor (TNF), interferon α, β, IL-1, IL-6 (Figure 2).11
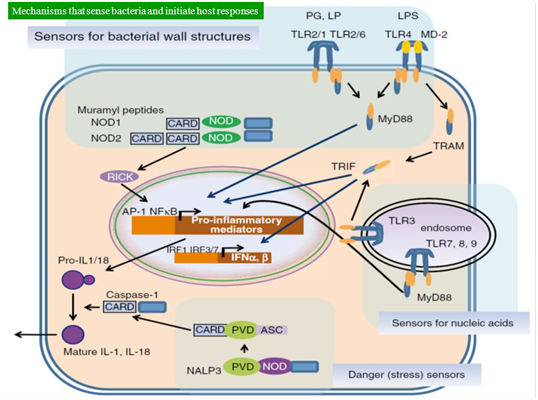
Figure 2 Mechanisms that sense bacteria and initiate host responses as adopted from Mendel’s Infectious Diseases.
In spite of the plethora of mediators released in face of an infective agent, the inflammatory response is suppressed in the blood stream. Not only to support the local defences but in order to prevent leucocyte-endothelial adhesion in uninvolved tissues and neutralize any chemical mediators escaping into the blood stream.12 Despite the above-mentioned interactions between pathogens and Toll- like receptors (TLRs), researchers have proved that TLRs genetically deficient mice still succumb to true models of sepsis.13 This in turn, proves that other pathogenetic mechanisms and other molecules are involved in this pathogen- host interaction.
It has always been believed that sepsis results from “excessive inflammation” and that blunting this exaggerated inflammatory response could save the lives of these patients.14 Consequently, several clinical trials were launched using anti-TNF and Interleukin-1 antagonists in the hope that blocking TNF or Interleukin-1 would improve survival in septic patients. Nevertheless, most of these studies did not show any significant or dramatic improvement in survival. Thus a search for other novel molecules could still be needed.14
On the other hand, there is another argument that states that septic patients fail to control the bacterial infection and die as a result of immunosuppression rather than immunostimulation. This has been emphasized by several studies that have shown that sepsis patients showed reduced production of both TNF and IL-6 whereas IL-10, the anti-inflammatory mediator, production remained high indicating a blunted inflammatory response.15,16 These data have justified the use of interferon-γ and granulocyte macrophage colony stimulating factor in various clinical trials to reverse the blunted monocyte response and thereby increase survival.17,18 These data indicate the inflammatory response in septic patients is quite complex and not exactly defined as exaggerated or blunted.
Sepsis and coagulopathy
The classic Virchow’s triad consists of hypercoagulability, endothelial injury and abnormal blood. In septic patients all these three alterations are present leading to activation of the extrinsic coagulation pathway (Tissue factor-dependent pathway).19 The production of thrombin via this pathway enhances platelet and endothelial activation as well as vascular smooth muscle changes leading to fibrin deposition and formation of vascular microthrombi ultimately leading to disseminated intravascular coagulopathy (DIC). The development of DIC in severe sepsis is a predictor of multiorgan failure and death in these patients.20
In a healthy human being, these effects are counteracted by Antithrombin III, Protein C, Protein S and tissue factor pathway inhibitor which are all reduced in severe sepsis thus favouring a prothrombotic antifibrinolytic state. Protein C has been identified as a major player in both the inflammatory and the coagulation response in cases of sepsis.21 In healthy human beings, Protein-C is activated by combination of thrombin and thrombomodulin, an endothelial cell surface protein (Figure 3).
When endothelial cells are injured, the extrinsic pathway of coagulation starts. First, tissue factor (TF) is released into the plasma and binds factor VIIa. This complex activates factor IX into IXa and factor X into Xa. Factor IX also converts factor X into Xa in the presence of co-factor VIIIa. Factor Xa converts pro-thrombin (Factor II) into thrombin (factor IIa) in the presence of co-factor Va. Thrombin then converts fibrinogen into fibrin which cross links the platelets resulting in platelet aggregation with consequent coagulation. All coagulation factors are in green, whereas the co-factors are in grey shades. This coagulation cascade is under strict control. First plasminogen is converted into plasmin in the presence of tissue plasminogen activator (t-PA). Plasmin then degrades fibrin (blue colors), and this axis is under the control of tissue plasminogen activator inhibitor (t-PAI). In addition thrombin is inhibited by anti-thrombin and TF is inhibited by TF pathway inhibitor (TFPI). Further, thrombin activates protein C which inhibits Factors V and VIII (purple colors). However, during septic shock, the release of inflammatory cytokines such as IL-6, TNF-α and IL-1β leads to blocking of TFPI, anti-thrombin and to increase the concentration of t-PAI (red colors). All these activities results in more coagulation and in the development of DIC.
Activated protein-C (APC) dissociates from its own receptor, endothelial protein C receptor (EPCR), binds soluble protein S to produce a complex that inactivates Factor Va and factor VIIIa thereby blocking the activation of thrombin. During acute phase, Protein C becomes depleted leading to impairment of Protein-C dependent anticoagulation, which is key to the development of thrombotic complications in septic individuals.12
Several large-scale multicenter clinical trials have contributed to the development of recombinant activated Protein C (APC) that was approved by the food and drug administration (FDA) for the treatment of sepsis. Later on, it has subsequently proven ineffective and was withdrawn from the market.19
Microvascular alterations and perfusion heterogeneity
In septic patients, the severity of organ dysfunction is always out of proportion of the degree of microcirculatory dysfunction represented by the mean arterial blood pressure and the cardiac output.12 However, alterations in the microcirculatory blood flow, in the form of decrease in capillary density, are now more recognized in severe sepsis and they are significantly correlated with the outcome.22–24 These alterations in capillary density leads to a dynamic heterogeneity of perfusion, meaning that capillaries that have no flow at a given time may become perfused a few minutes later and vice-versa.
In a study by De Baker and colleagues,22 it has been documented that microcirculatory perfusion is significantly altered in patients with septic shock and in another study24 it was shown to be an independent factor associated with survival. This heterogeneity in perfusion is associated with heterogeneity in tissue oxygenation and the development of hypoxic zones even when the blood flow to the organ is preserved thus contributing to cellular and organ dysfunction in patients with septic shock.25
Central to these alterations is again “endothelial dysfunction”. There are alterations in the glycocalyx, the thin protein on the surface of the endothelial cell that contains antithrombin and superoxide dismutase. There is also a decrease in is size25–27 and increase in its products of degradation in the blood.26,28 This in turn leads to leukocyte rolling and adhesion to the surface of the endothelium as well as impairing the circulation of RBCs leading to impaired release of nitrous oxide (NO).
From the above-mentioned data, we may conclude that several mechanisms are involved in the microvascular alterations and there is no single agent that may prove effective in reversal of this process.
Mitochondrial dysfunction/cytopathic hypoxia
Microcirculatory alterations are not the sole contributor to cellular dysfunction, it has been suggested that mitochondrial dysfunction plays an important role as well. The term “cytopathic hypoxia” was proposed to describe diminished oxygen utilization with resultant decreased production of ATP even if cellular oxygen concentration is normal. The decreased ATP production is associated with increased free radical production and depletion of the anti- oxidant reserve. In sepsis, part of this oxidative burst is triggered by macrophages leading to increase in expression of inducible nitric oxide synthase (iNOS) with more NO production leading to inhibition of mitochondrial function.29
Therefore, it was quite logical to try and reverse that oxidative stress by giving these patients anti-oxidants. Several clinical trials using anti-oxidants like N- omega-methyl-L-arginine or even selective inhibitors of iNOS have been done but none gained FDA- approval so far.30,31
A recent advance in this particular area, was suggested by Bar-O and colleagues who suggested that sepsis/SIRS followed by treatment with O2 and mechanical ventilation was equivalent to ischeamia- reperfusion model created by Chouchani in 2014. In their study they suggested that in ischemia, which is usually present in sepsis, there is catalysis of fumarate to succinate by succinate dehydrogenase enzyme, also known as complex II, resulting in accumulation of succinate in mitochondria during ischemia. When they treated sepsis by O2 and mechanical ventilation, this mimics the reperfusion in Chiouchani model resulting in massive production of free radicals that overwhelm the endogenous anti-oxidants supply adding to the oxidative stress in the mitochondria and contributing to progressive mitochondrial dysfunction. The suggestion to administer oxygen in sepsis patients accompanied by mitochondrial redox reagents to eliminate the free radical burst32 needs to be validated.
None.
The authors declare there is no conflict of interests.
None.

©2016 Guimei, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.