MOJ
eISSN: 2373-4442


Research Article Volume 6 Issue 1
Defence Institute of Physiology and Allied Sciences (DIPAS), India
Correspondence: KP Mishra, Immunomodulation Laboratory, Defence Institute of Physiology & Allied Sciences, Lucknow Road, Timarpur, Delhi, India,, Tel 911123883162, Fax 91 11 23932869
Received: January 01, 1971 | Published: January 5, 2018
Citation: Sharma N, Mishra KP, Ganju L, Singh SB (2018) Salidroside Exerts Anti-Inflammatory Effect by Reducing Nuclear Factor ?B and Nitric Oxide Production in Macrophages. MOJ Immunol 6(1): 00183 DOI: 10.15406/moji.2018.06.00183
Objective:Chronic inflammation is an underlying cause of wide variety of human pathologies. In the present study we evaluated the anti‒inflammatory properties of salidroside, a major bioactive component of Rhodiola rosea and possible underlying mechanisms related to its anti‒inflammatory activity.
Materials and methods:We investigated the effect of salidroside on nuclear markers, iNOS, NFκB, IκB and pIκB. LPS induced nitric oxide (NO) production in mouse peritoneal macrophages, RAW 264.7 cells and primary mixed glial cells and expression of inflammatory cytokines measured by ELISA. In addition the action of salidroside on adjuvant induced paw edema, a mouse model of arthritis was also determined.
Results:Our results showed that salidroside significantly decreased the expression of NFκB and pIκB in LPS induced peritoneal macrophages from mice. It also significantly decreased NO and iNOS production in peritoneal macrophages, RAW 264.7 and primary mixed glial cells after LPS treatment. Salidroside was found to decrease production of pro‒inflammatory cytokines:TNF‒α, IL‒1β and IL‒6. In vivo, salidroside significantly decreased paw‒edema in a mouse model of arthritis.
Conclusion:The significant decrease in LPS induced NFκB, pIκB, iNOS, NO production and pro‒inflammatory cytokines by salidroside indicated that salidroside exhibits activities consistent with anti‒inflammatory mechanisms and may have utility as a therapeutic agent in treating or managing inflammatory diseases.
Keywords:Inflammation, Salidroside, NFκB, NO, Pro‒inflammatory cytokines
NF‒κB, Nuclear Factor Kappa‒Light‒Chain‒Enhancer of Activated B Cells; NO, Nitric Qxide; TNF‒α:Tumor Necrosis Factor α; IL‒1β, Interleukin‒1β; RA, Rheumatoid Arthritis; IBD, Inflammatory Bowel Disease; COPD, Chronic Obstructive Pulmonary Disease; IKK‒1, Inhibitor of Kappa B Kinase; ROS, Reactive Oxygen Species; INOS, Inducible Nitric Oxide Synthase; AIA:Adjuvant Induced Arthritis; DEX, Dexamethasone; CFA, Complete Freund’s Adjuvant
In the human immune system, inflammation, a complex biological, non‒specific immune response against noxious stimuli such as irritants, pathogens and damaged cells initiates the process of healing.1 Inflammation is a first line of defense against foreign intruders playing a critical role in early recognition and subsequent stimulation of pro‒inflammatory response to the infectious organisms. Chronic inflammation, in contrast, forms a major cause of debilitating human diseases like rheumatoid arthritis (RA), inflammatory bowel disease (IBD), asthma, atherosclerosis, periodontitis, hay fever, chronic obstructive pulmonary disease (COPD) and some forms of cancer also. Transcription factor NFκB is a master regulator of genes involved in inflammatory responses. Activation of NFκB can be initiated by, for example, agonists of TLRs (Toll‒like receptors) such as LPS or pro‒inflammatory cytokines such as IL‒1, IL‒8, IL‒12, IFN‒γ and TNF‒α. A further downstream event in inflammation also involves activation of the canonical NF‒ΚB pathway.2 Aupperle et. al. reported that activation of NFκB is governed by IκB kinases, IKK‒1 and IKK‒2 in fibroblast like synoviocytes isolated from synovium of rheumatoid arthritis and osteoarthritis patient.3‒5 Activation of NFκB causes proinflammatory cytokines to be produced in human atherosclerotic plaques.6 NF‒κB activation has also been reported in inflammatory disease such as arthritis7 and allergic airway disease.8
Activation of NF‒κB induces production of nitric oxide (NO) and reactive oxygen species (ROS). Nitric oxide is involved at almost every stage of inflammation, particularly the early stages of inflammatory cell migration to sites of inflammation.9 IFN‒γ, TNF‒α or IL‒1β stimulate production of inducible Nitric oxide synthase (iNOS). However, the most extensively studied inducer is lipopolysaccharide (LPS). In response to certain cytokines (TNF‒α, IL‒1β) and some other inflammatory mediators, the production of relatively large quantities of NO is stimulated. In larger quantities, NO is a potent vasodilator, helps in macrophage‒induced cytotoxicity, and may result in joint destruction in some types of arthritis. In the present study we examined the effects of salidroside on inflammation and the mechanism by which it exerts its effects.
Salidroside (p‒hydroxyphenethyl‒β‒D‒glucoside, C14H20O7), is a major active component of Rhodiola rosea L. (Crassulaceae). Rhodiola rosea plant mainly grows in high altitude region of arctic Europe and Asia.10 Extensive toxicological studies have been performed on this plant and it has been certified safe for both animals and humans.11 Rhodiola rosea. is characterized as an adaptogen, this feature is attributed mainly to its two compounds p‒tyrosol and the phenolic glycoside, salidroside (Figure 1).12 A variety of antioxidant compounds have been identified in Rhodiola rosea including p‒tyrosol, organic acids (gallic acid, caffeic acid, and chlorogenic acid), and flavonoids (catechins and proanthocyanidins.13 It has been also observed to have anti‒inflammatory, neuroprotective and antiviral properties.14,15 Salidroside is found in phenyl ethanol derivatives fraction of Rhodiola rosea.16 It has many other pharmacological properties like antioxidative, antidepressant, antifatigul, cardio protective and neuroprotective properties.17‒20 Recently Sharma et al. reported the activity of salidroside against dengue fever.21 However, little information is available in the literature about the anti‒inflammatory effects of salidroside,22 so in the present study we determined the anti‒ inflammatory activity of salidroside in LPS induced peritoneal macrophages.
Animals
Thirty, 5‒6 week old male BALB/c mice weighing 22‒25 g were housed per cage in the animal care facility of the Defense Institute of Physiology and Allied Sciences (Delhi), with access to food and water ad libitum. All the experiments were performed in accordance with the regulations specified by the Institute's Animal Ethical Committee and conform to the National Guidelines on the Care and Use of Laboratory Animals, India.
Reagents
Salidroside (purity 99.8%) was purchased from Sigma‒Aldrich Fluka (Cat.No. 43866). LPS (Escherichia coli 026:B6) was purchased from Sigma (Cat.No. L‒2654, USA). Mouse tumor necrosis factor (TNF)‒α and interleukin (IL)‒6 enzyme‒linked immunosorbent assay (ELISA) kits were purchased from eBioscience (CA,USA). Rabbit anti mouse NFκB antibody was purchased from Bio vision (Cat.No. 3038, CA,USA), iNOS (Cat.No. SAB 4502011), pIκB (Cat. No. SAB 4503880), IκB (Cat.No. SAB4501994), Beta actin (Cat.No. A3854, SIGMA), goat anti rabbit immunoglobulin (IgG) biotinylated (Cat.No. B8895 Sigma) and streptavidin peroxidase (Cat.No. S2438 Sigma) were purchased from Sigma, USA.
Cell viability assay using MTT dye
Mice were injected intraperitoneally with one ml thioglycollate (4%) 3 days prior to sacrifice for the isolation of peritoneal macrophages. Animals were euthanized and peritoneal macrophages were collected by injecting and repeated flushing with ice cold autoclaved PBS inside the peritoneal cavity. The cells were washed once with PBS and suspended in RPMI‒1640 medium supplemented with 10% FBS. The cell viability assay was performed using 3(4, 5‒dimethyl thiazole ‒2‒yl)‒2‒5, diphenyl tetrazolium bromide (MTT) dye. Briefly, peritoneal macrophages were isolated and cultured at 0.5 × 106/ml respectively in a 96 well plate. Peritoneal macrophages were treated with salidroside at concentrations of 33, 83,166, 333 & 666 nM for 24 h, 48 h and 72 h. The MTT dye was added and incubated further for 4 h at 37 °C at 5% CO2. The formazan crystals formed in the live cells were solubilized using DMSO and the absorbance was measured at 570 nm.
Determination of effect of salidroside on inflammatory factors by immunoblotting
The peritoneal macrophages (PMφ) isolated from individual animals were incubated for 24 h at 37 °C, 5% CO2 and then washed with ice cold PBS and finally, treated with whole cell lysis buffer (10 mM Tris‒base, 5 mM EDTA, 50 mM NaCl, 1% Triton X‒100, and 10 μl protease inhibitor cocktail). The cells were lysed on ice for 30 min with intermittent vortexing at every 10 min interval. The cell lysates were centrifuged at 12000 rpm for 5 min and the supernatants obtained were collected and estimated for protein concentration using Bradford's reagent. Anti NF‒kB antibody was used to probe the nuclear extract proteins, Antibodies against iNOS, pIκB, IκB and beta actin were used to probe whole cell lysate proteins. The membranes were then washed three times for 10 min with TBST20 and incubated with the secondary antibody, goat anti rabbit IgG biotinylated and streptavidin peroxidase. The proteins were detected by chemiluminescence.
Nitric oxide estimation
The nitric oxide levels were estimated using Greiss reagent by the protocol originally described by Greiss. The peritoneal macrophages collected as described above, were added into 96 well plates at a concentration of 0.5 × 106 cells/ml with or without ex‒vivo LPS (1 μg/ml) treatment and salidroside treatment. The plate was incubated at 37°C, 5% CO2 for 48 h. After incubation, nitrite production in the cell supernatants was determined using Greiss’reagent. The optical density of the supernatant was measured at 540 nm using a spectrophotometer. The nitrite concentration in each sample was calculated using a standard curve prepared by serial dilution of known concentration (in μM) of sodium nitrite (NaNO2).
Cytokines estimation by ELISA
The serum samples collected from individual mice were analyzed for the levels of pro‒inflammatory cytokines (TNF‒α and IL‒6) by a sandwich ELISA following manufacturer's instructions. Briefly, the 96 wells plates were coated with capture antibodies specific for mouse TNF‒α and IL‒6 and incubated overnight at 4 °C. The plates were washed five times for 1 min each with wash buffer (0.01 M PBS, pH‒7.0 and 0.05% Tween 20) and blocked using assay diluent (provided in the kit) for 1 h, followed by a further five washes and then the respective standards and samples were added in duplicates and incubated for 2 h at room temperature. The respective detection antibodies were added for 1 h and plates were washed as described earlier followed by addition of avidin‒HRP for 30 min and washed. Color was developed by adding TMB substrate following incubation of 15 min. The reaction was stopped by adding 2 N sulphuric acid and the absorbance was measured at 450 nm. The respective cytokine levels in samples were calculated from a plot of standards.
Reverse transcription polymerase chain reaction (RT‒PCR)
Total cellular RNA was extracted from peritoneal macrophages using an RNeasy Protect Mini Kit (QIAGEN, Germany), following the manufacturer’s instructions. Amplification of the target gene was performed using a One‒step RT‒PCR Kit (QIAGEN Germany), with specific primers, following manufacturer’s protocol. The primer pairs and their product sizes were as follows; beta actin:forward primer, 5′ATATCGCTGCGCTGGTCGTC‒3′; reverse primer, 5′AGGATGGCGTGAGGAGAGC‒3′, bp. IL‒1β:forward primer, 5′GCAACTGTTCCTGAACTCA‒3′; reverse primer, 5′CTCGGAGCCTGTAGTGCAG‒3′, bp. The IL‒1β and Beta ‒actin PCR products were analyzed by electrophoresis on a 2 % agarose gel.
Real‒ time reverse transcriptase‒ polymerase chain reaction (RT‒ PCR)
Total RNA was obtained from peritoneal macrophages as previously described by RNeasy Protect Mini Kit (QIAGEN, Germany). Total RNA was further processed into cDNA using Verso cDNA synthesis kit (Thermofisher Scientific, USA). Real‒time PCR (qPCR) of IL‒1β and GAPDH was carried out using the prepared cDNA as a template using SYBER Green by BIORAD CFX connect instruement. GAPDH was used as a housekeeping gene. Mouse IL‒1β specific primers were designed with the Xcelris labs limited (Gujrat, India). The sequences of IL‒1β primers for quantitative RT‒PCR are 5′GCAACTGTTCCTGAACTCA‒3′; reverse primer, 5′CTCGGAGCCTGTAGTGCAG‒3′, GAPDH forward primer, 5′CCATGTTCGTCATGGGTGTGAACCA‒3′; reverse primer, 5′ GCCAGTAGAGGCAGGGATGATGTTC‒3′. Cq values of the indicated genes in each sample were calculated, and the fold change in gene expression of IL‒1β in comparison to the house keeping gene were calculated by 2‒ΔΔCq method.
Adjuvant induced arthritis (AIA) mouse model and treatment regimen
Thirty male BALB/c mice, weighing 25‒28 gram were divided into five groups of six mice where the first group was the control group, the second group of mice received Complete Freund’s adjuvant (CFA) only, the third group of mice were treated with both CFA and salidroside at 83µM/kg, the fourth group of mice were treated with both CFA and salidroside at 166µM/kg, and the fifth group of mice received CFA and dexamethasone (DEX) as a positive anti‒ inflammatory control. Both Salidroside (83µM/kg and 166µM /kg) and DEX (0.5 mg/kg) were injected intra peritoneally. The untreated control mice were injected intraperitoneally with an equal volume of PBS. To develop AIA, the mice were injected with 0.2 ml of CFA (Sigma Chem¬icals, St. Louis, MO, USA) mixed with phosphate buffer saline in the ratio 1:1 into the right hind paw using the uninjected left hind paw as a control. Measurements were obtained from both the inflamed and noninflamed hind limb paws on day‒1, 3, 5,7,9,11,13 and 15. Salidroside and DEX treatment were given to the mice from day 3 after CFA injection on day 1, for three consecutive days.
Statistical analysis
The differences between LPS and salidroside + LPS treated groups and controls were analyzed using a commercially available statistics software package (SPSS for Windows, version 14.0, Chicago, USA). A one‒way analysis of variance (ANOVA) test was performed. Differences were considered significant at p ≤ 0.05. Data was presented as Mean ± SEM (Standard Error).
Cell viability assay
The cytotoxicity of salidroside to peritoneal macrophages was tested using a MTT assay. Treatment of cells with salidroside at concentration of 33,83 and 166 nM resulted in no cytotoxicity in peritoneal macrophages up to 48 h of incubation (Figure 2). A higher dose, 333 nM produced up to 15% cell cytotoxicity. Therefore, we selected nontoxic doses of salidroside (33, 83 and 166 nM ) to test its anti‒inflammatory activities.

Figure 2 Cytotoxicity of salidroside in peritoneal macrophages. Readings at 24h, 48h and 72h time point were conducted in triplicate.
Effect of salidroside on LPS induced expression of NFκB, IκB and p IκB
As measured by immunoblotting of nuclear and whole cell lysates of peritoneal macrophages, salidroside significantly decreased pIκB expression (87 fold) (Figure 3), (p<0.0001) and NFκB (6.8 fold) (Figure 3A and 3D), (p<0.0001) There was a marked increase in IκB expression (2.2 fold) (Figure 3A and 3C) (p<0.0001) in salidroside treated and LPS stimulated group in comparison to LPS alone treated group.
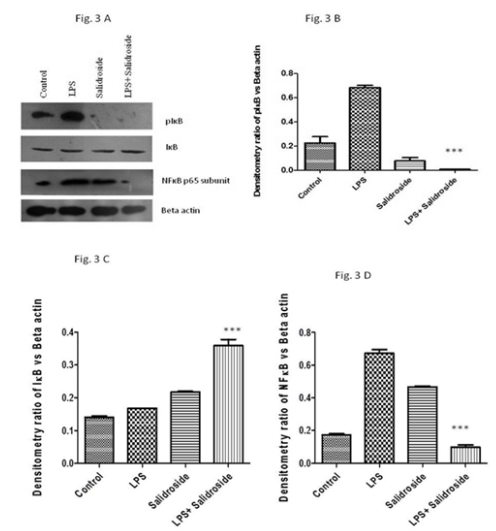
Figure 3 Effect of salidroside on inflammatory mediators. A:Immunoblot of pIκB, IκB, NFκB and beta actin in LPS stimulated PMφ on salidroside treatment; B:Bar diagramme of density ratio of pIκB versus beta‒actin in LPS stimulated PMφ on salidroside treatment. *p<0.0001; C:Bar diagramme of density ratio of IκB versus beta‒actin in LPS stimulated PMφ on salidroside treatment *p<0.0001; D:Bar diagramme of density ratio of NFκB versus beta‒actin in LPS stimulated PMφ on salidroside treatment *p<0.0001.
Effect of salidroside on LPS induced NO and i NOS production
As compared to LPS, salidroside (166 nM) produced no increase in spontaneous release of NO after 48 h in the supernatants of peritoneal macrophages of individual animals (LPS 72 μM vs salidroside 22 μM) (Figure 4A), (p<0.0001). The reduction in NO by salidroside was higher using 166 nM than for 33 or 83 nM, so was chosen for additional experiments. Similarly the in vitro data in the RAW cell line and primary mixed glial cells also demonstrated an inhibitory effect of salidroside on nitric oxide release from LPS treated macrophages as salidroside decreased NO from 18 μM to 10 μM and 37 μM to 2.4 μM respectively (Figure 4B & 4C), (p<0.0001). In addition salidroside treatment significantly decreased iNOS expression levels 2.4 fold in comparison to the LPS stimulated peritoneal macrophages (Figure 4D & 4E), (p<0.01).

Figure 4 Effect of salidroside on NO and iNOS production.
A:NO secretion in mouse peritoneal macrophages (PMφ) *p<0.0001; B:NO secretion in RAW cell line *p<0.0001; C:NO secretion in primary mixed glial cells *p<0.0001; D:Immunoblot of i‒NOS expression in LPS stimulated PMφ after salidroside treatment; E:Bar diagramme of density ratio of i‒NOS versus beta actin. *p<0.01 The results shown are representative data from three independent experiments.
Effect of salidroside on LPS induced pro‒inflammatory cytokines
Production of pro‒inflammatory cytokines in supernatants from LPS stimulated peritoneal macrophages as measured by ELISA showed a marked decrease in the LPS induced cytokines TNF‒α and IL‒6 in salidroside treated samples as compared to the LPS only samples (Figure 5A & 5B). Salidroside also significantly decreased IL‒1β expression at the transcription level (Figure 5C & 5D), (p<0.0001). Further, salidroside decreased IL‒1β expression by 19 fold as measured by real time PCR (Figure 5E), p<0.05).

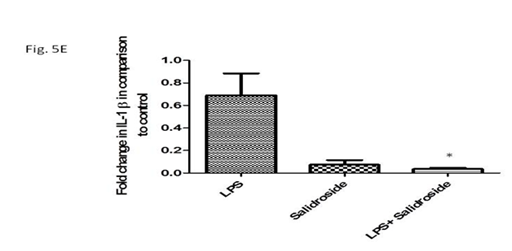
Figure 5 Effect of salidroside on cytokine release.
A:TNF‒α secretion in cell supernatant obtained from LPS stimulated and salidroside treated cells by ELISA; B:IL‒6 secretion in cell supernatant obtained from LPS stimulated and salidroside treated cells by ELISA; C:Expression of IL‒1β m‒RNA in LPS stimulated and salidroside treated PMφ; D:Bar diagramme showing density ratio of IL‒1β versus Beta –actin *p<0.0001; E:Bar diagramme showing Fold change in IL‒1 β in comparison to control by real time PCR.*p<0.05.
Effect of salidroside on adjuvant induced inflammation
The anti‒inflammatory effects of salidroside in vivo were evaluated by using the initial response to adjuvant in the hind paw of a mouse. In this study, mice developed edema and swelling in the injected (right) hind paw within 4 h after the administration of complete Freund’s adjuvant increasing from 1.5 mm up to 6 mm by the second day. Two doses of salidroside treatment (83 µM/kg and 166 µM/kg) were given from day 3 after CFA injection on day 1. Salidroside treatment significantly decreased paw thickness to 3.367±0.185 mm in group administered 83 µM/kg group on day 5 in comparison to CFA group (5.51±0.29, p<0.001). A similar pattern of decrease in paw thickness was observed in 166 µM/kg salidroside group of mice. It was observed that 166 µM/kg dose of salidroside significantly reduced CFA induced paw edema to 3.2±0.152 mm on day 5 in comparison to CFA group (5.51±0.29, p<0.001) and finally to 1.73±0.03 mm on day 15. This decrease in paw thickness was slightly even more than positive control dexamethasone which reduces paw thickness to 3.76±0.142 on day 5 in comparison to CFA group (5.51±0.29, p<0.001) and finally to 1.86±0.03 on day 15 (Figure 6).
As our immune system becomes overburdened with pathogens, irritants and damaged cells, inflammatory triggers are cycled continuously through the blood where they affect nerves, organs, connective tissues, joints, and muscles. The inflammatory response to these triggers can lead to numerous serious and debilitating diseases. The use of medicinal plants as complementary therapies is gaining momentum in recent times and more importantly in developing countries where the cost of drugs makes their use unaffordable. Repurposing of already‒known botanical remedies can provide alternatives to the development and use of novel anti‒inflammatory compounds. In this study we observe that salidroside is a potent anti‒inflammatory drug which can be used as therapeutic agent against various inflammatory diseases. To characterize the nature of the inhibitory effect of salidroside on inflammatory mediators, we examined the effects of salidroside on the activation of the transcription factor NF‒κB, which is a master regulator of inflammation. NF‒κB is essential for host defense and inflammatory responses to microbial and viral infections.23,24 In the majority of cells, NF‒κB exists in an inactive form in the cytoplasm, bound to the inhibitory IκB proteins. Treatment of cells with various inducers results in the degradation of IκB proteins via their phosphorylation and subsequent translocation of NF‒κB p65 into the nucleus, where NF‒κB activates appropriate target genes. In the present study, our results indicated salidroside treatment upregulated I𝜅B and downregualated phosphorylated IκB in LPS stimulated peritoneal macrophages which in turn inhibited the activation of NF‒𝜅B. We speculate that the attenuation of the severity of inflammation by salidroside treatment is mediated by suppression of inflammatory responses through downregulating phosphorylation of NF‒𝜅B activity. LPS, is a well known bacterial endotoxin, which triggers deleterious systemic inflammatory responses when released into blood circulation, which can lead to septic shock and cause organ damage.25 In the present study we observed that salidroside significantly decreases LPS induced NO production not only in PMφ but also in primary mixed glial cells suggesting it may have affect neuroinflammation also. It also decreases NO production in RAW 264.7 cell line confirming its potent anti‒inflammatory activity. As NO is involved in the pathogenesis of inflammatory disorders of the joints, gut and lungs, NO inhibitors may represent a strategy in the management of inflammatory diseases.26
It is well known that pro‒inflammatory cytokines induced by LPS, such as TNF‒α, IL‒6 and IL‒1β, play a key role in the process of inflammatory diseases. Excessive production of these and other pro‒inflammatory cytokines results in a systemic inflammatory response syndrome, such as septic shock. Based on this information, the pharmacological inhibition of these inflammatory mediators has been an important target in pursuing treatment for diseases characterized by chronic inflammation. Among the inflammatory cytokines, TNF‒α plays a key role in regulating inflammation, mostly through the induction of other inflammatory cytokines including IL‒1 (IL‒1α and IL‒1β), IL‒6, IL‒8, granulocyte‒macrophage colony‒stimulating factor and adhesion molecules.27 Salidroside treatment was found to decrease expression of TNF‒ α, and IL‒6, which further supports our hypothesis. IL‒1 β has been proposed as the key cytokine involved in the pathogenesis of osteoarthritis, because it induces inflammatory reactions and catabolic effects independently as well as being combined with other mediators with respect to the articular cartilage and other elements of joints.28 In this study we observed that salidroside treatment significantly decreases expression of IL‒1 β an excellent anti‒inflammatory property of salidroside. Injection of adjuvant (Mycobacterium tuberculosis inactivated) in mice produces an immune reaction that characteristically involves inflammatory destruction of cartilage and bone of distal joints with concomitant swelling of surrounding tissues.29 In this study, salidroside was found to significantly decrease paw edema in the early stages of model of arthritis in the mouse. This decrease in paw thickness was commensurate with or greater than that of dexamethasone, the positive anti‒inflammatory control. As the in vivo data supported in‒vitro data, there is good evidence that salidroside has anti‒inflammatory activity. In summary, salidroside exhibited mechanistic activity that may have excellent anti‒inflammatory potential as evidenced by suppressing LPS induced NO production and decreasing the expression of i NOS, NF‒𝜅B, pI𝜅B and down regulating pro‒inflammatory cytokines. It also reduced adjuvant induced paw‒thickness in mice. These data support the potential therapeutic use of this agent to treat inflammatory diseases.
Authors thank Defence Research & Development Organization (DRDO), Ministry of Defence, Government of India, for financial support in the form of project DIP‒264. NS thanks Council of Scientific and Industrial research for providing the fellowship in the form of junior and senior research fellowship.
Authors have no conflict of interest.

©2018 Sharma, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.