MOJ
eISSN: 2373-4442


Research Article Volume 5 Issue 1
Defence Institute of Physiology & Allied Sciences (DIPAS), India
Correspondence: Richa Rathor, Defence Institute of Physiology & Allied Sciences (DIPAS), Lucknow Road, Timarpur, Delhi-110054, India, Tel 91-11-23946257, Fax 91-11-23914790
Received: January 01, 1971 | Published: January 24, 2017
Citation: Rathor R, Meena DK, Shyam R, Misra K (2017) Immunostimulatory Activity Investigation of Aqueous and Hydroethanolic Extract of Wheatgrass Using THP1 Cells. MOJ Immunol 5(1): 00146. DOI: 10.15406/moji.2017.05.00146
Wheatgrass, Triticum aestivum is well known for its high nutritional values. However the immunomodulatory activity of the same is unexplored. Therefore, the present study was planned to elucidate the immunomodulatory activity of aqueous and hydro–ethanolic extract of wheatgrass. For immunomodulatory activity analysis, THP1 cells were treated with and without lipopolysaccharide (LPS) and aqueous and hydroethanolic wheatgrass extract (50µg/mL and 100µg/mL, respectively) for 24 h. After 24 h, supernatants were harvested and cytokine assays were performed. NO production in terms of amount of nitrite content was also estimated in treated and untreated murine peritoneal macrophages using Griess reagent. Further, a dose dependent in–vitro comparative study concluded that both the extracts possess immunostimulatory activity. However comparison between both established that aqueous extract of wheatgrass having more immunostimulatory activity at the dose of 100μg/ml as extract significantly upregulated LPS induced release of TNF–α, IL–1β and IL–6 in THP1 cells. The extract also significantly induced nitrite release in macrophage cells without causing any toxic effects. The findings suggest the non–specific immunostimulatory activity of wheatgrass; hence it could be used in enhancing immune responses and disease resistance.
Keywords: immunomodulation, wheatgrass, triticum aestivum, cytokines, thp1 cells, lipopolysaccharide, immune responses, disease resistance, thp1 cells
WG1: Aqueous Extract of Wheatgrass; WG2: Hydro–Ethanolic Extract of Wheatgrass; C: Control; LPS: Lipopolysaccharide; FBS: Fetal Bovine Serum; PBS: Phosphate Buffered Saline; NO: Nitric Oxide; TNF–α: Tumor Necrosis Factor– α; DMSO: Dimethyl Sulfoxide; ANOVA: Analysis of Variance; NCCS: National Centre for Cell Sciences
Immunomodulation means the ability to alter immune system of an organism via interfering its functions. Enhancement of immune system is known as immunostimulation which primarily implies stimulation of non–specific system; on the other hand, immunosuppressant implies reduction of resistance against infections and stress. Immunostimulation and immunosuppression both activities require for regulating the normal immunological functioning. Therefore both immunostimulating and immunosuppressing have their own importance and search for better agents exerting these activities is becoming the field of major interest all over the world.1–2
From the ancient time, plant products were used as immunostimulant or immunosuppressant for cure and remedy of humans. Beside this, these plant based product also used as the principal content drugs.3–4 Recently, a growing attraction was observed towards the use of plant/herbal products as an effective alternative therapy for the prevention and treatment of numerous medical problems as plant products have advantages over the conventionally used drugs, which are not only expensive but also have harmful side effects.5,6
Wheatgrass basically is young grass of the common wheat plant that belongs to Poaceae family. Commonly, it is edible in India, prepared from cotyledons of the common wheat plant, Triticum aestivum. Triticum aestivum may be consumed as fresh juice or dried powder by animal and human. It is believed that wheatgrass is having full nutritional value which is cause of having antioxidant, hypocholestrolemic, anti–carcinogenic, laxative, astringent, diuretic, antibacterial and anti–aging properties.7–10 Beside these properties, wheatgrass is also used for number of conditions like common cold, cough, bronchitis, fever, infections, inflamed mouth and throat, and skin disorders like hemorrhoids, psoriasis, ivy, eczema, burns and thalassemia.11 In spite of having numerous beneficial properties, a limited research has commenced to provide its immunomodulatory activity. To keeping these facts in our mind, the present comparative study was planned to investigate the immunomodulatory activity of aqueous and hydro–ethanolic extract of wheatgrass using in–vitro model, LPS–stimulated human monocytic THP1 cells.
Chemicals
RPMI and fetal bovine serum (FBS) were purchased from HiMedia, India. TNF–α, IL–1β, IL–6 and NF–κB ELISA kits were purchased from Cayman Chemicals, New Orleans, Louisiana, USA. Lipopolysaccraide (LPS) was purchased from Sigma, USA.
Plant material
Wheatgrass was procured from Grime’s WG, Sholapur, Maharashtra, India. The characterized commercially available wheatgrass was used in the present study. Leaves of wheatgrass was clean twice with distilled water followed by it was dried in clean, shaded and dust free area. Dry leaves were crushed by laboratory blander and further used for extraction process.
Preparation of aqueous and hydro–ethanolic extract of wheatgrass
Accelerated solvent Extraction system ASE 350 equipped with a solvent controller unit from Dionex Corporation (Sunnyvale, CA, USA) was used for the extraction. Extraction was carried in 33 ml extraction cells, containing 2gm of sample in triplicate using millipore water and 70% ethanol as a solvent (for aqueous and hydro–ethanolic extract respectively) at 25°C±2°C for 15 min. The heat–up time changed according to extraction temperature and is automatically fixed by the equipment.
In vitro immunomodulatory activity
Cell line and tissue culture media: THP1 cell line was obtained from National Centre for Cell Sciences (NCCS), Pune, India. Cells were grown in RPMI 1640 media containing 25mM HEPES, 2mM l–glutamine (Sigma, St. Louis, MO), 10% heat inactivated fetal bovine serum, penicillin (100 U/ml), and streptomycin (100ug/ml) in a humidified atmosphere of 5% CO2 at 37°C.
MTT assay for cell viability
The cell viability was tested using yellow tetrazolium salt, MTT.12 The assay was performed in 96–well tissue culture plates. In brief, THP–1 cells (2X105 cells/ml) were incubated for 24 h in a humidified atmosphere of 5% CO2 at 37°C with varying concentration of aqueous and hydro–ethanolic extract of wheatgrass in the presence and absence of LPS (0.2 µg/ml). After incubation, 20 µl of 5mg/ml MTT solution was added and incubated for additional 4hr under the same conditions. Followed by, the supernatant were aspirated to an eppendorf tube from culture plate and centrifuged at 3200rpm at 4°C for 15 min. Supernatant was removed and the blue formazon crystals were solubilized in 200 µl of dimethyl sulfoxide (DMSO) under agitation. After dissolving the crystals, optical density were in a microtiter plate reader (BioTek, Powerwave XS2, USA) at 570 nm.
Determination of TNF–α, IL–6 and IL–1β
THP–1 cells (2X105cells/ml) were incubated with different concentrations of aqueous and hydro–ethanolic extract of wheatgrass in the absence and presence of LPS (0.2µg/ml) for 24 hrs in a humidified atmosphere of 5% CO2 at 37°C. Supernatant was collected and stored at –80°C until measurements of TNF–α, IL–6 and IL–1β was performed.
TNF–α, IL–6 and IL–1β levels in supernatant were determined by using commercial enzyme–linked immunosorbent assay (ELISA) kits (Cayman, USA) following manufacturer’s instructions.
Mouse peritoneal macrophages isolation and culture for measurement of nitrite concentration
To study the effects of wheatgrass extract on NO production, a griess reagent based method was used.13 Nitric oxide was measured as nitrite released from mouse macrophage cells. For isolation of macrophages cells, 2% starch suspension was prepared in phosphate buffered saline (PBS) and 1.5 ml of the above solution was injected into the peritoneal cavity of mice. After 3 days, peritoneal fluid was collected in the cold phosphate buffer saline (PBS). Collected peritoneal fluid was centrifuged at 400g for 10 min; the resulting pellet was suspended in complete RPMI1640 medium at a concentration of 5×105 cells per ml. The cells are allowed to adhere to the substrate by culturing them 2 hr at 37°C. Nonadherent cells are removed by gently washing three times with warm PBS. At this time, total cell population was 92%.
Mouse macrophages were cultured in RPMI1640 media with 25mM HEPES, 2mM glutamine, 10% fetal bovine serum, penicillin (100 IU/ml), and streptomycin (100 IU/ml). Mouse peritoneal macrophages were cultured with different concentrations of aqueous and hydro–ethanolic extract of wheatgrass with or without LPS in a humidified incubator at 37°C in 5% CO2 for 48hrs. NO production in supernatant was measured in terms of amount of nitrite, its stable product in a microplate assay. To measure nitrite, 100 μL of macrophage culture supernatant was collected, mixed with an equal volume of Griess reagent (A solution of sulfanilamide and N–(1–naphthyl)–ethylenediamine in 2M hydrochloric acid) and incubated for 10 min at room temperature. Nitrite concentration was determined by measuring the absorbance at 550 nm with a microplate reader. NaNO was used for external calibration.
Nuclear transcription factor – kB expression (NF–kB)
1.0X107cells/ml was suspended in RPMI 1640, centrifuged at 300Xg for 5 min at 4°C and the supernatant was discarded. Then, nuclear extract was prepared as per instructions given in Cayman’s Nuclear Extraction Kit, U.S.A. Then NF–kB was estimated by ELISA kit (Cayman, USA). All tests were performed in triplicates.
Statistical analysis
Data are presented as Mean ± SEM. One way Analysis of Variance (ANOVA) followed by Berferroni’s multiple comparisons test using Graph Pad Instat7 (Graph Pad Software Inc, La Jolla, CA). A p–value <0.05 was considered statistically significant.
In this study, human monocyte cell line was used to identify the immunomodulatory activity of wheatgrass (different extract and different concentration) using various immunomodulatory markers (proinflammatory cytokines, TNF–α, IL–1β and IL–6) in unstimulated and LPS–stimulated THP–1 cells.14
Effect of aqueous and hydro–ethanolic extract of wheatgrass on cell viability
In the same experiment, we also evaluated if the stimulatory effect on cytokine release due to direct upregulated effect of THP1 cells by aqueous and hydro–ethanolic extract of wheatgrass. The viability of THP1 cells were measured by MTT assay.
No significant change was observed in unstimulated, stimulated and wheat grass treated cells which demonstrated that viability was unaffected by using aqueous and hydro–ethanolic extract of wheatgrass (Figure 1).
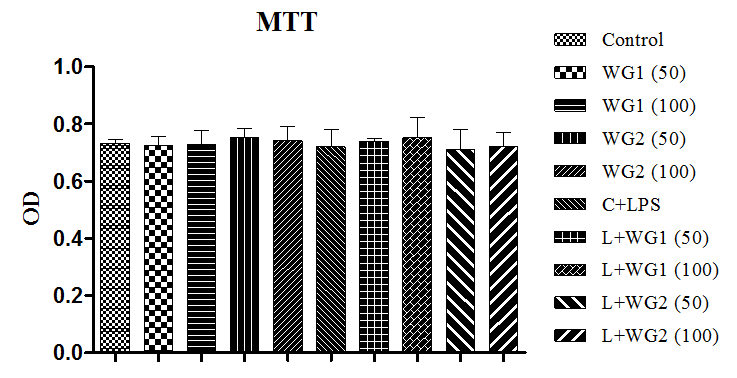
Figure 1 Effect of aqueous and hydro-ethanolic extract of wheatgrass cell viability in THP1 cells.
WG1: Aqueous extract of wheatgrass; WG2: Hydro-ethanolic extract of wheatgrass; C+LPS: Lipopolysaccharide treated; L+WG1: Lipopolysaccharide +Aqueous extract of wheatgrass treated; L+WG2: Lipopolysaccharide+Hydro-ethanolic extract of wheatgrass treated. Data represents Means±SEM of three independent experiments carried out in triplicates.
Effect of aqueous and hydro–ethanolic extract of wheatgrass on TNF–α release
Tumor necrosis factor–α (TNF–α), also known as cachectin, plays roles in antitumor activity, immune modulation, inflammation, anorexia, cachexia, septic shock, viral replication, and hematopoiesis.15,16 The results depicted TNF– α production increased significantly in LPS treated cells in comparison to control cells. TNF– α secretion was also increased once cells were treated with LPS and WG1 at the dose of 100 µg/ml (2756±48.87pg/ml, p<0.05). Beside this, WG1 alone (100 µg/ml) also significantly increased TNF–α level (358±10.42pg/ml) (Figure 2).
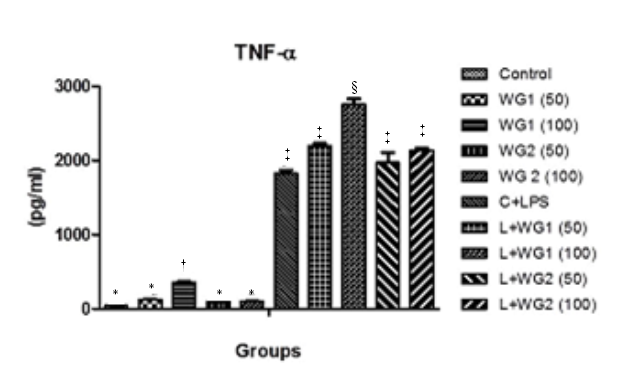
Figure 2 Effect of aqueous and hydro-ethanolic extract of wheatgrass on the production of TNF-α in THP1 cells.
WG1: Aqueous extract of wheatgrass; WG2: Hydro-ethanolic extract of wheatgrass; C + LPS: Lipopolysaccharide treated; L+WG1: Lipopolysaccharide+Aqueous extract of wheatgrass treated; L+WG2: Lipopolysaccharide+Hydro-ethanolic extract of wheatgrass treated.
*,†,‡,§Differences between values with matching symbol notations within each column are not statistically significant at 5% level of probability. Data represents Means±SEM of three independent experiments carried out in triplicates.
Effect of aqueous and hydro–ethanolic extract of wheatgrass on IL–1β release
IL–1β significantly increased in LPS treated THP1 cells in comparison to control (36.94±0.61 pg ml and 288.6±8.91pg/ml respectively). WG1 extract at the dose of 50 and 100 µg/ml was able to increase more IL–1β secretion in LPS treated cells. Beside this, WG1 alone at the dose of 100µg/ml also increased IL–1β (59.07±3.868pg/ml, p<0.05) (Figure 3).
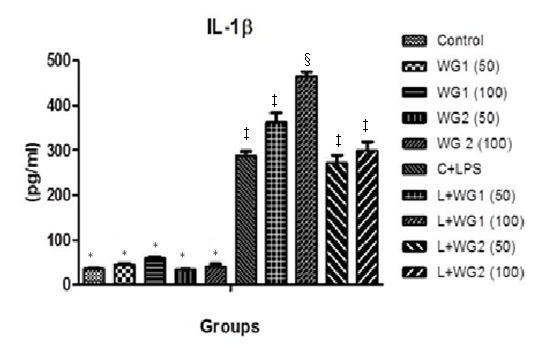
Figure 3 Effect of aqueous and hydro-ethanolic extract of wheatgrass on the production of IL-1β in THP1 cells.
WG1: Aqueous extract of wheatgrass; WG2: Hydro-ethanolic extract of wheatgrass; C + LPS: Lipopolysaccharide treated; L+WG1: Lipopolysaccharide +Aqueous extract of wheatgrass treated; L+WG2: Lipopolysaccharide+Hydro-ethanolic extract of wheatgrass treated.
*,†,‡,§Differences between values with matching symbol notations within each column are not statistically significant at 5% level of probability. Data represents Means±SEM of three independent experiments carried out in triplicates.
Effect of aqueous and hydro–ethanolic extract of wheatgrass on IL–6 release
A significant increment of IL–6 was observed in LPS treated THP1 cells compared with control cells (5.23±0.472pg/ml and 212.3±6.339pg/ml respectively). The increase of IL–6 was also increased significantly once cells were only treated with WG1 (100 μg/ml) (Figure 4).
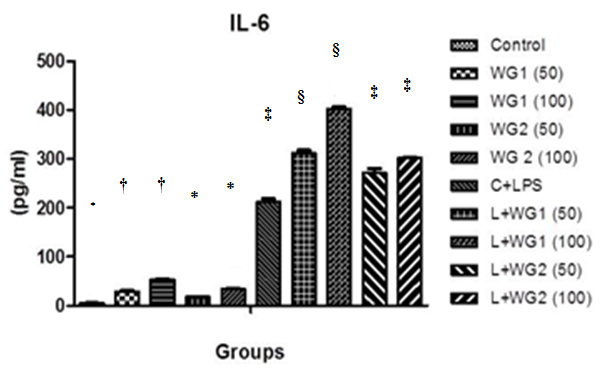
Figure 4 Effect of aqueous and hydro-ethanolic extract of wheatgrass on the production of IL-6 in THP1 cells.
WG1: Aqueous extract of wheatgrass; WG2: Hydro-ethanolic extract of wheatgrass; C + LPS: Lipopolysaccharide treated; L+WG1: Lipopolysaccharide +Aqueous extract of wheatgrass treated; L+WG2: Lipopolysaccharide+Hydro-ethanolic extract of wheatgrass treated.
*,†,‡,§Differences between values with matching symbol notations within each column are not statistically significant at 5% level of probability. Data represents Means±SEM of three independent experiments carried out in triplicates.
Effect of aqueous and hydro–ethanolic extract of wheatgrass on NF–κB expression
NF–κB expression increased significantly in LPS exposed cells. With the support of other parameters, the expression of the same increase significantly in cells treated with higher dose of WG1 (100μg/ml) with or without LPS (Figure 5).
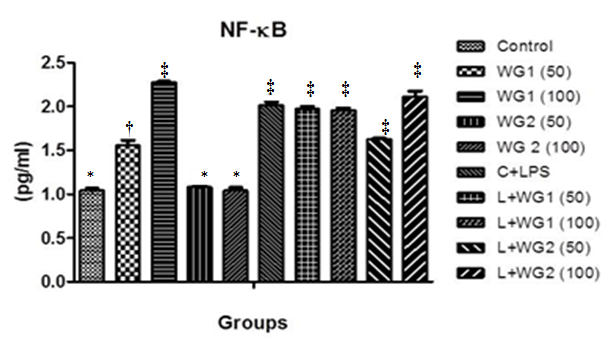
Figure 5 Effect of aqueous and hydro-ethanolic extract of wheatgrass on the production of NF-κB in THP1 cells.
WG1: Aqueous extract of wheatgrass; WG2: Hydro-ethanolic extract of wheatgrass; C + LPS: Lipopolysaccharide treated; L+WG1: Lipopolysaccharide +Aqueous extract of wheatgrass treated; L+WG2: Lipopolysaccharide+Hydro-ethanolic extract of wheatgrass treated.
*,†,‡Differences between values with matching symbol notations within each column are not statistically significant at 5%level of probability. Data represents Means±SEM of three independent experiments carried out in triplicates.
Effect of aqueous and hydro–ethanolic extract of wheatgrass on nitrite production
Nitrite production was increased significantly in LPS treated THP1 cells compared with control cells. The increase in NO was increased more after treatment with 100μg/ml of WG1 (Figure 6).
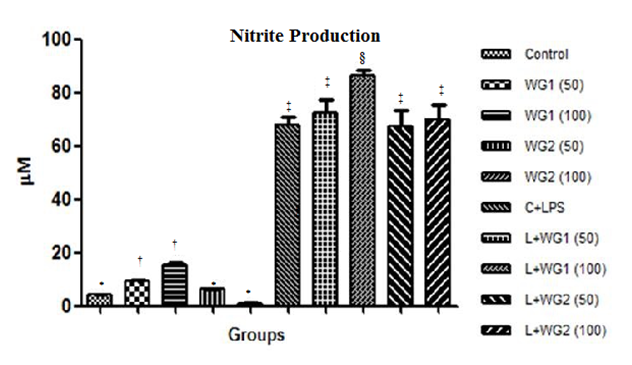
Figure 6 Effect of aqueous and hydro-ethanolic extract of wheatgrass on nitrite production in THP1 cells.
WG1: Aqueous extract of wheatgrass; WG2: Hydro-ethanolic extract of wheatgrass; C + LPS: Lipopolysaccharide treated; L+WG1: Lipopolysaccharide +Aqueous extract of wheatgrass treated; L+WG2: Lipopolysaccharide+Hydro-ethanolic extract of wheatgrass treated.
*,†,‡,§Differences between values with matching symbol notations within each column are not statistically significant at 5% level of probability. Data represents Means±SEM of three independent experiments carried out in triplicates.
The present study gives an overview of immunomodulatory activity of wheatgrass, a popular herb of India with special reference to pro–inflammatory cytokines (viz TNF–α, IL–1β and IL–6 expression) and NF–kB activity using monocytic cell line THP1. Khan et al.17 conducted a chromatographic analysis of wheatgrass and described the presence of gallic acid and rutin as nutritional supplement in the same extract.
The present study made a comparison between aqueous and hydro–ethanolic extract of wheat grass in view of immunomodulatory activity. Interestingly, we found that both the extract possessed immunostimulatory activity but aqueous extract of wheatgrass was having more immunostimutory activity as compared with hydro–ethanolic extract of wheatgrass.
The first response towards infection is inflammation which used to start with the production of pro–inflammatory cytokines like TNF–α, IL–1β and nitric oxide (NO). Thus inhibitors of these cytokines could be considered as immune–suppressive drug while enhancers of these cytokines could be known as immune–stimulators. The widely distributed key mediators of inflammation are monocytes/macrophages.18 Along with this, endotoxin or other external stimuli activates monocytes/macrophages which also engage to produce pro–inflammatory cytokines like TNF–α, IL–1β and IL–6.19 Beside the cytokines cascade, other inflammatory mediators such nitric oxide (NO) and NF–kB also play an important role during inflammation.20,21 Nitric oxide (NO) is a signaling molecule which used to play a prime role in pathogenesis of inflammation. Under normal condition, it gives anti–inflammatory effect while during abnormal situation, it used to consider as pro–inflammatory mediator that induces inflammation, vasoconstriction and tissue damage.22 Nitric oxide (NO) is a signaling molecule which used to play a prime role in pathogenesis of inflammation. Under normal condition, it gives anti–inflammatory effect while during abnormal situation, it used to consider as pro–inflammatory mediator that induces inflammation, vasoconstriction and tissue damage.22 Nuclear factor kappa B (NF–kB), a transcription factor involved in regulating various genes for cytokines, chemokines, adhesion targets and acute phase proteins. Beside this, NF–kB is also responsible for growth, development, apoptosis and immune/immunomodulatory responses.21 The present results showed upregulation of pro–inflammatory cytokine levels such TNF–α, IL–1β and IL–6. Along with this, nitrite production, indicator of nitric oxide and NF–kB was also found increased during aqueous wheat grass extract treatment.
The finding of the present study demonstrated that wheatgrass is a potent activator of TNF–α, IL–1β, IL–6 and nitrite production in LPS–stimulated THP1 cells. Moreover, this induction of immune response was also associated with NF–kB activation due to wheatgrass. Beside this immune–stimulant role of wheatgrass was not associated with any cell death as no significant change was observed in MTT assay at any dose of wheatgrass extract on LPS stimulated and unstimulated THP1 cells. In other word, it could also be state that cell viability was not affected due to wheatgrass treatment (Figure 7). Conclusively, the present study depicts non–specific immunostimulatory activity of wheatgrass. Non–specific immunostimulant used to enhance innate or non–specific immune response via interacting and activating immune cells. Another well known fact regarding non–specific immunostimulants that it do not contain antigen specific activity and are widely used in chronic infections, immunodeficiency, autoimmunity and neoplastic diseases. Immunostimulants can be grouped under different agents based on the source, such as bacterial preparations, polysaccharides, animal or plant extracts, nutritional factors and cytokines.23,24 Based on these facts wheatgrass could also be used in enhancing immune responses and disease resistance.
None.
None.

©2017 Rathor, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.