MOJ
eISSN: 2373-4442


Review Article Volume 2 Issue 4
1Department of Immunology, School of Medicine, Kerman University of Medical Sciences, Iran
2Department of Urology, Bahonar Hospital, Kerman University of Medical Sciences, Iran
3Department of Immunology, Sirjan Faculty of Medical Sciences, Iran
Correspondence: Mohammad Sadegh Razeghi, Department of Immunology, Sirjan Faculty of Medical Sciences, Kerman University of Medical Sciences, Kerman, Iran, Tel 9194159280
Received: October 02, 2015 | Published: October 16, 2015
Citation: Mohammadi MM, Ketabchi AA, Razeghi MS (2015) Immunological Aspects of Varicocele. MOJ Immunol 2(4): 00054. DOI: 10.15406/moji.2015.02.00054
Varicocele is the most common cause of infertility in men. The mechanism by which varicocele cause the variable effect on male infertility and is still unknown. Varicocele is found in 35 to 81 % of infertile men, and is one treatable form of male infertility. Although many advances have occurred in the treatment of varicocele, it still represents an important and challenging aspect of basic research (male reproductive physiology and endocrinology, pathophysiology, and pharmacology of reproduction and fertility) and medical practice for urologists, pediatric surgeons, and general physicians, to date. This review provides an overview of the epidemiology, Clinical classification and Immunological aspects of varicocele and discusses the indications for, and interpretation of Immunological aspects of varicocele.
Keywords, infertility, varicocele, ASA, OS, apoptosis, Fas/Fas-L system
ASA, antisperm antibody; Ig, immunoglobulin; IL, interleukin; OS, oxidative stress; ROS, reactive oxygen species; RNS, reactive nitrogen species; NO, nitric oxide; TAC, total antioxidant capacity; WHO, World health organization; SP, seminal plasma; TNF, tumor necrosis factor; Fas-L, fas-ligand; Sfas, soluble fas
Infertility is considered as one of the main public health issues, because as it affects about 15% of the couples of reproductive age. The male factor is involved in 40-50% of infertility cases.1,2 Primary male infertility is defined as never being able to initiate conception, whereas secondary male infertility is defined as having been able to father children in the past but currently having difficulty.3 Varicocele is an abnormal dilation of the pampiniform venous plexus in the scrotum that develops during puberty Figure 1; it can affect testicular growth and semen parameters, and is considered to be a major cause of male infertility.4–10 Clinical varicoceles cause some problems from cosmetically to erectile dysfunction and infertility.11,12 Although the incidence of varicocele in the male general population is roughly 15%, it has been implicated as a factor responsible for infertility in as much as one-third of the infertile population.13 Incidence of varicocele is increased by about 10% for each decade of life and reaches up to 75% above the eighth decade.14 Varicocele is found in approximately 15% of the general population, in 35% of men with primary infertility, and in 70% to 81% of men with secondary infertility.5,15,16–20 This review provides an overview of the epidemiology, Clinical classification and Immunological aspects of varicocele and discusses the indications for, and interpretation of Immunological aspects of varicocele.
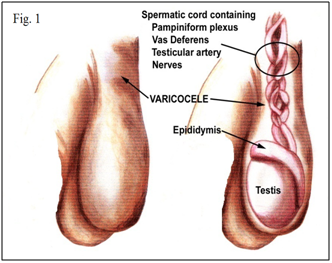
Figure 1 Varicocele is an abnormal dilation of the pampiniform venous plexus in the scrotum that develops during puberty.
Clinical classification of varicocele
The clinical severity of the disease is divided into three degrees. In grade 1, the enlargement of the venous plexus of spermatic tone is evident only by palpation during the Valsalva manoeuvre. In grade 2, the enlargement of the venous plexus of spermatic tone is evident only by palpation at upright position. Grade 3 is visually evident and is easily diagnosed by physician.1,7 The exact mechanism by which varicocele affects male fertility and spermatogenesis is unknown Clearly, the factors contributing to abnormal sperm function caused by varicocele that lead to infertility are ambiguous.21,22 Several hypotheses have been raised to explain the mechanisms by which scrotal varicocele may exert a deleterious effect on spermatogenesis and male fertility. They include renal and adrenal reflux, hypoxia, hormonal dysfunction, hyperthermia, and apoptosis of germ cells.9 Varicocele is asymptomatic in most cases and the diagnosis is made during physical examination by scrotal palpation. When symptoms are observed, they are usually loss of testicular mass, pain, and scrotal size asymmetry.10 Varicocele is unilateral and left sided in at least 85% of cases. In most of the remaining cases, the condition is bilateral. Unilateral right-sided varicocele is rare.23 Although they probably originate due to valvular absence or incompetence in the internal spermatic veins, varicoceles may occur due to a number of different mechanisms, and is thus considered a multifactorial disease.10,24
Varicocele and autoimmunity
The blood-testis barrier immunoregulatory proteins at the level of the sertoli cells, ret testis and efferent ductules, provide immunological protection of sperm antigens and inhibit lymphocyte proliferation and compelmant-mediated cell lysis. Disruption of this blood-testis barrier is believed to result in the production of antisperm antibodies. The proposed aetiologies for such disruption include ductal obstructions, testicular torsion, infection/epididymitis, prostatitis, testicular trauma and varicocele.25 Antisperm antibodies (ASA) have been identified in 9% to 12.8% of infertile couples, in 1% to 2.5% of fertile men, and in 4% of fertile women. Different isotopes of ASA have been identified, specifically immunoglobulin (Ig) A, IgG, and IgM. Whether or not a varicocele leads to development of ASA remains controversial.26 Autoantibodies to sperms are present approximately in 10% of infertile males and in (24.6% and 32%) among patients with varicocele. However, a number of investigators have found no association between ASA formation and varicocele. ASA impair the fertilizing ability of spermatozoa by acting negatively on spermmotility and result in poor cervical mucus penetration and in vitro gamete interaction. Sperm-bound antibodies have been found to impair sperm function only when the degree of antibody binding is very high (>50%).27 In an animal model of unilateral varicocele, researchers reported that significant titers of ASA were present in animals with varicoceles but not in controls.28 Hooman Djaladat et al.29 indicated that varicocelectomy may reduce the ASA level, and this reduction has a good effect on semen parameter quality. Varicocelectomy procedure, however, may raise the ASA level in some patients albeit without significant adverse effect to semen parameters.
Oxidative stress leads to cellular dysfunction Figures 2 & 3. Because of the high cellular turnover in normal spermatogenesis, Reactive oxygen species (ROS) such as superoxide, hydroxyl, peroxyl, hydroperoxyl and Reactive Nitrogen species (RNS), such as NO and nitrogen dioxide, which are produced by the per oxidation and oxidation of many cellular lipids, proteins, carbohydrates and nucleic acids, are generated. The plasma membrane of testicular cells is rich in polyunsaturated fatty acids, and is therefore vulnerable to oxidative stress.6,30 In normal healthy men, the seminal plasma (SP) contains natural scavengers or antioxidants to neutralize the effects of excessive ROS/RNS generation. Under pathological conditions, however, ROS/RNS production overwhelms the antioxidant capacity and causes increased oxidative stress.31–34 ROS/RNS may cause defective sperm function as a result of lipid per oxidation of the polyunsaturated fatty acids in the sperm head and mid-piece, alter sperm morphology and lead to decreased motility and ineffective spermatozoon-oocyte fusion reaction.35–40

Figure 2 Oxidative stress is essential to maintain cellular homeostasis, but excess oxidative stress leads to cellular dysfunction.
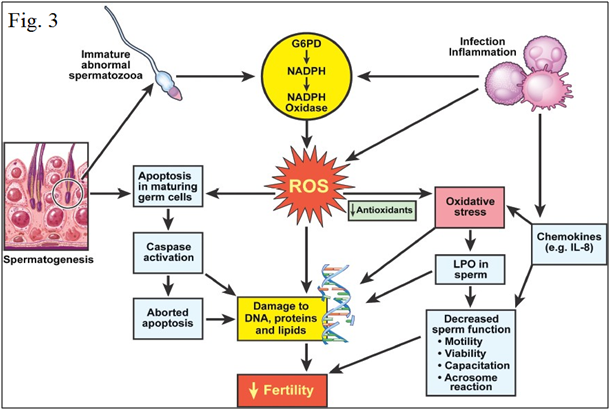
Figure 3 Mechanism of oxidative stress in human semen.40
Varicocele and leukocytes
Measurement of leukocytes in semen has been a standard component of the semen analysis, but its true significance is still unknown.41 Leukocytospermia defined by the World Health Organization (WHO) as more than 1 million leukocytes per millilitre.42 Leukocytospermia is thought to have multifactorial origin. In addition to genital tract infections, other etiologies such as smoking, alcohol consumption, and marijuana use increase WBC in semen.43 Some studies found no detrimental effects of Leukocytospermia44,45 but several others correlated seminal leukocytes with impaired semen parameters, especially sperm morphology and motility.46–48 Adding to the confusion is an older study that suggested that seminal leukocytes at concentrations between 1 and 3 million/mL (M/mL) are beneficial for sperm function, arguably due to effects of cytokines or scavenging of abnormal sperm.49 Increased WBC in semen can be noticed in men with abnormal spermatogenesis as protective mechanism for the removal of defective sperm from the ejaculate. Finally, varicocele or vasovasostomy can result in high number of leukocytes in semen.50 Tortolero et al.51 reported that increased number of semen lymphocytes is more frequent in subfertile and varicocele males than in fertile males. The increase of semen leukocytes is associated with deterioration of seminal parameters. Oxidative stress has a negative influence on seminal parameters in subfertile males of unknown etiology.
Varicocele and cytokines
The cytokines are a group of soluble mediators produced by lymphoid and non lymphoid cells that play a key role in the afferent and efferent phases of immune responses of both the innate and acquired immune systems.52 Spermatogenesis depends upon numerous local signaling molecules, which provide integration and communication among the different cell types in the testis during hormonal regulation of germ cell maturation. This local regulatory control is supported by a large number of cytokines.53 Infection of the seminal ducts can lead to male infertility by various mechanisms. Direct damage may be caused by microorganisms and/or their secretory products, while secondary inflammation may further increase the number of activated leukocytes and an increased secretion of cytokines.54 The presence of interleukins (ILs) and several other cytokines in seminal plasma was reported in multiple studies in the literature.54,55 Interleukin-6 (IL-6) is a multifunctional cytokine found in seminal fluid that is produced by a number of different cells.56 Infertile patients with varicocele have greater levels of cytokines and oxidative stress than healthy patients as indicated by elevated levels of IL-6 and ROS and decreased levels of total antioxidant capacity. Pro-inflammatory cytokine IL-6 and oxidative stress may contribute to the pathophysiology of the infertility in men with varicocele.57
Tumor necrosis factor (TNF), formerly known as Tumor necrosis factor alpha is now regarded as a natural component of the mammalian seminal plasma. Although not completely clarified, its functions in the SP have been associated with paradoxal roles, such as sperm survival in the female genital tract; while at high levels negatively affect sperm survival and fertility potential.58 Papadimas et al.59 suggested that the determination of IL-1b and TNF-a in human spermatozoa does not provide useful information in male routine infertility workup. Nevertheless, a better understanding of these mediators in semen of normal men and patients with andrological diseases may contribute to a new approach to the management of male infertility. Eggert-Kruse et al.60 reported that in patients with varicocele median TNF-a levels was lower in these men compared with the other patients without reaching significance.
Varicocele and apoptosis
It is suggested that the spermatogenic dysfunction in varicocele testis may be related partly to an abnormal control of apoptosis.13,61,62 Apoptosis, or signal-induced cell death, is a process in which a genetic mechanism, responsible for a series of events related to morphologic and biochemical changes, initiates by certain stimuli and culminates in the death of a cell. It is an important mechanism through which altered or excessive cells are removed from a population, maintaining tissue integrity, differentiation, and characteristics.63,64 There are two major pathways that lead to apoptosis, an intrinsic, mitochondria-initiated pathway and an extrinsic pathway which initiates upon the activation of membrane death receptors.65–67 The Fas Protein (APO-1 or CD95) and its ligand (Fas-ligand or Fas-L) are members of the tumor necrosis factor (TNF) family. When Fas binds to Fas-L, a molecular complex is formed, signaling initiation of apoptosis, which involves caspase 8 activation and a subsequent cascade of events leading to DNA fragmentation and cell death.68–70
In the testis, Fas may be found in spermatogenic germ cells, and Fas-L in Sertoli cells.13,69,71 It seems that the Fas/Fas L system is a major regulator of normal spermatogenesis.72 The Fas system in the testis has been identified as a paracrine signaling system by which Sertoli cells, expressing Fas-L, can initiate. reported that the level of seminal ROS correlates with the varicocele grade in men with this disease.37 Specifically, men with grade 2 and 3 varicocele have greater levels of ROS in the seminal plasma than men with grade 1 varicocele.38 Sharma et al.39 indicated that among 56 varicocele patients, ROS concentrations were higher and the total antioxidant capacity (TAC) was lower than those of the control group. Although most studies show that seminal ROS levels are higher in men with varicocele than controls, others question the association between varicocele and the seminal ROS level.6,21 Indeed, Cocuzza et al.21 reported that the presence of clinical varicocele in fertile men is not associated with higher seminal ROS concentrations. Levels of ROS are not correlated with varicocele grade or testis volume in the same population of fertile men.
Varicocele and oxidative stress
In the past 10 years, oxidative stress (OS) has been the most investigated factor involved in the pathophysiology of varicocele. Oxidative stress is essential to maintain cellular homeostasis, but excess killing of Fas expressing germ cells. In addition, soluble forms of cell surface receptors such as soluble Fas (sFas) can be produced either by proteolysis cleavage of membrane-bound receptors or by alternative splicing and it is believed to inhibit Fas-Fas L binding and thereby block Fas-mediated apoptosis.73,74 Sakkas et al.,75 first described the presence of Fas on ejaculated sperm and proposed the “abortive apoptosis” theory states that an apoptotic process begins in germ cells but fails to be completed and deleted, can end up as Fas positive sperm in the semen. They also proposed that this theory may explain the presence of abnormal sperm observed in semen samples. In support of this theory, Sakkas et al.76 showed that 10–50% Fas expression in oligoasthenoteratozoospermia and oligoteratozoospermia samples were significantly higher than the percentage of Fas positive sperm in men with normal semen parameters.77 In contrast, later studies apparently have reached opposite conclusions which showed that Fas protein was not detected on the ejaculated sperm of normozoospermic and non-normozoospermic men.78,79
Fujisawa et al.,80,81 indicated that apoptosis was decreased in the testes of patients with varicocele compared with those of controls. Contrarily, Fujisawa and Ishikawa demonstrated increased apoptosis in oligozoospermic patients with varicocele having lower seminal sFas than patients without varicocele or fertile men. They addressed the relationship between varicoceles and apoptosis by monitoring the concentrations of the soluble form of Fas (sFas) in seminal plasma, to characterize the Fas signalling system with regard to hypospermatogenesis as the result of varicocele. Recognizing that sFas could block Fas-dependent apoptosis, they screened the seminal plasma of oligospermic men with varicoceles, oligospermic men with no varicocele, and normal controls, for the levels of sFas and the sFas ligand. SFas were not detected in any of the cases, whereas sFas levels were specifically lower only in cases of varicocele. These reduced sFas levels were reversed by varicocelectomy. However, although higher temperatures may inhibit sFas production in patients with varicocele the reason for this decrease in sFas levels remains unknown. Celik-Ozenci et al.,61 detected that expression of Fas-L in spermatids was significantly down-regulated after induction of experimental varicocele. Giudice et al reported that in adolescents with varicocele presenting lower sperm concentration, Fas-L mRNA levels are higher than in adolescents without varicocele showed that apoptosis is increased in ejaculated spermatozoa in patients with varicocele compared to normal fertile men.71
Previous studies showed that varicocele had negative effect on spermatogenesis and observed DNA fragmentation of sperm cells in these patients. Sperm DNA damage, independent of its cause, may affect the quality of the ejaculated spermatozoa and may have implications on their fertility potential. A number of studies have proposed that the presence of spermatozoa with damaged DNA may be the result of an impaired chromatin packing or may be indicative of apoptosis. Nevertheless, the major cause of this negative effect on sperm is not clear. Both basic and clinical studies are required to investigate this condition to improve spermatogenesis.
None.
The authors declare that there are no conflicts of interest.
None.

©2015 Mohammadi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.