MOJ
eISSN: 2373-4442


Review Article Volume 5 Issue 7
1epartment of Dermatology and Clinical Immunology, University of Saint Francis Xavier, Bolivia
2Department of Infectious Disease, Sulaiman Al Habib Hospital, Saudi Arabia
Correspondence: Fabiola Ramallo Jadue, abiola Ramallo Jadue, Department of Dermatology And Clinical Immunology, University of Saint Francis Xavier, Bolivia
Received: January 01, 1971 | Published: November 1, 2017
Citation: Jadue FR, Guevara JA (2017) Immunological aspects of Hansen’s Disease. MOJ Immunol5(7): 00182. DOI: 10.15406/moji.2017.05.00182
Considering the complexity of the clinical manifestations of Hansen's Disease and its correlation with the immunological alterations found within the clinical aspect of the disease. Leprae defense is the response to cell-mediated immunity, especially macrophages and T lymphocytes, which by means of the effective interaction of their activities, may or may not destroy the bacilli. All the evolution and pathophysiology of the disease may be related to the immunological alterations of the parasite - host reaction that occurs in leprosy. After the presentation of the characteristic immunological problems and their correlation with the clinical forms they become fundamental for the understanding of the disease.
Keywords: Clinical Immunology, Immunity, Immunological alterations, T lymphocytes, Pathophysiology, Immunological problems
Leprosy is a serious public health problem for causing permanent physical disability. It is caused by Mycobacterium Leprae, obligate intracellular parasite, is characterized by presenting with a broad spectrum of clinical manifestations, which contain at their ends polar opposites and stable, interspersed by unstable forms, which may acquire clinical and immunological aspects of each of the poles, depending on the host immune response potential (Figure 1).
Mycobacterium Leprae, the first bacterium described as pathogenic to man, was discovered by Amauer Hansen in 1873. It was found in the form of sticks, acid resistant alcohol (BAAR), when stained with red by acid fuchsin (Ziehl Neelsen) on lesion frescoes Inhabited, has very slow reproduction (12 to 14 days) and affinity for cutaneous and peripheral nerve cells, preferably located in colder regions of the human body such as the nose, testicles and places where the nerves are very close to the skin Like the lobes of the ears.1
The multibacillary disease (HV, DV, DD) is the main source of infection because it has a high bacillary load in the dermis and in mucous membranes. It is accepted that leprosy is transmitted through the airways. There is no evidence to date that the transmission is by airway, another possibility of transmission, may be cutaneous, when there are ulcerated lesions or trauma to the skin.2,3 As for the influence of environmental factors, the transmission of the disease exists practically only in tropical countries, coinciding with an underdevelopment, whose poverty is configured as a risk factor. The importance of nutritional status, household overcrowding or the presence of other concomitant diseases in the onset of leprosy is unknown. That is why the interaction between intrinsic factors of the individual (genetic susceptibility) and environmental factors is fundamental. Little is known of the genetic susceptibility of this infection although it seems that the majority of the population (about 90%) is genetically resistant, by mechanisms still unknown. Those who develop the disease go through long periods of incubation (3 to 5 years), is followed by the initial phase of Leprosy and is not contagious, does not leave sequels.
TT: Tuberculoide
BT: Borderline Tuberculoide
BB: Borderline Borderline (Indetermined)
BL: Borderline Lepromatosa
LL: Lepromatosa (Table 1)
TT |
BT |
BB |
BL |
LL |
|
Number of Injuries |
Usually Unique |
One or Few |
Several |
Numerous |
Very Numerous |
Size |
Variable |
Variable |
Variable |
Variable |
Small |
Surface |
Very Dry Sometimes Flaky |
Dry |
Discretely Bright |
Bright |
Bright |
Sensitivity |
Absent |
Markedly Diminished |
Moderately Diminished |
Slightly Diminished |
Can be Affected |
Piloso Growth |
Absent |
Markedly Diminished |
Moderately Diminished |
Slightly Diminished |
May Not be Affected |
Baar in Injury |
Do Not |
Not O Few |
Moderately |
Many |
Globi |
Baar in Moco Nasal |
Do Not |
Do Not |
Do Not |
Generally Not |
Very Numerous |
Lepromina |
+++ |
+ Ó ++ |
Negative |
Negative |
Negative |
Table 1 The initial phase of Leprosy and is not contagious, does not leave sequels
Immunological responsiveness can be assessed by the Mitsuda Reaction, which consists of the intradermal inoculation of heat-killed bacilli (human or tattooed), which is read after four weeks, resulting in infiltrated papules, Positive reaction or, absence of skin alteration, negative reaction. The result of this reaction associated with bacillary load may signal the patient's immune responsiveness. Thus, those susceptible to this disease have a high number of bacilli (mediated by the bacilloscopic index and absence of immune response (Mitsuda negative), as for the resistant ones there is immunological hyperactivity (Mitsuda positive) and absence of bacilli in the lesions. Dimorphic forms can strike a balance between these two factors.4,5
The evaluation of the humoral response shows activation of this in Virchowians and DV, characterized by high levels of specific antibodies (Anti PGL1), high concentrations of PGL1 antigen in peripheral blood that reflect the accentuated bacillary load of these patients. In the Tuberculoid the levels of this antibody appear similar to the two normal controls. Anti PGL1 and antibody specific for the antigen PGL1 glycolipid phenolic wall M. leprae, and does not cross-react with M. tuberculosis or other mycobacteria. The humoral response is ineffective for the removal of bacilli in HV, the defense efficacy is effected by cells capable of phagocytizing and destroying the bacterium (Macrophages - APC), presenting only in its antigenic fraction the effector cells.6
The presence of acyl within macrophages induces its activation resulting in the production of IL-1, TNF-alpha and IL-2 cytokines, which act on T lymphocytes, usually a CD4 + phenotype (Helper) population, making them activated and With the capacity to produce their own cytokines. The IL-2 cytokine directly stimulates the NK (Natural Killer) cell, which is now producing INF-gamma. This cytokine, INF-gamma. This cytokine INF-gamma, stimulates the macrophage and associated with the action of TNF both act synergistically, increasing macrophage activation. T lymphocyte (TH1 subpopulation), produces the cytokines IL2, INF and TNF beta, proinflammatory cytokines, responsible for the maintenance of immunocellular response.7 The TH2 subpopulation produces cytokines IL4, IL5, IL6, IL8 and IL10 are suppressors of macrophagic activity.
Therefore, depending on the subpopulation of T cells and macrophagic activity, there will be predominance of defense mechanisms or disease dissemination, expressed clinically by the tuberculoid and virchowian form respectively. The mechanisms of defense are related to the presence of the cytokine TNF alpha and INF gamma and to the production of oxidation mediators, such as intermediate oxygen reactants (ROI) and Nitrogen (RNI) intermediates, essential elements for the bacillary destruction inside the Macrophage. But in some situations where there is production of the PGL1 and LAM (lipoarainomannan) antigens, the bacillus may present escape mechanisms to intramacrophagic oxidation (Figure 2).
The destruction or multiplication of the bacillus inside the macrophage can be determined by immunological mechanisms that involve the presentation of the antigen (MHC COMPLEX) and HLA histocompatibility antigen, both genetically inherited. In HT there is a predominance of the HLA-DR2 and HLA-DR3 phenotype of HLA antigens, not conferring susceptibility to the disease, in HV and DV predominantly the HLA-DQ1 phenotype, related to the susceptibility. The specific difference in the ability of virchowian macrophages to destroy M. leprae is associated with these factors. The relationship between immunological rivalry and the type of HLA antigen highlights that macrophages, infected by the bacillus are one of the elements that can lead to the type of evolution of the infection. It was observed that HV macrophages produced significantly lower amounts of TNF alpha and IL-6 compared to HT production, suggesting that macrophagic response depression is associated with the presence of the bacillus and / or its components (Figure 3).
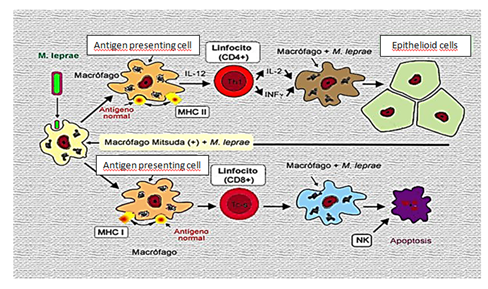
Figure 3 The macrophagic response depression is associated with the presence of the bacillus and / or its components.
In macrophages of animals infected with M. leprae a reduction in the ability to produce oxygen derivatives, superoxide anion and resistance to the action of gamma INF was observed in the activation of these cells. As well as in cell cultures of patients with leprosy developed in the presence of M. Leprae and its derivatives: PGL1 and LAM. The mechanisms involved in this reaction favor the bacillary multiplication associated with the release of cytokines blocking the macrophagic activity (IL-10 and TGF-beta and the development of specific suppression of the immune system of Virchowians, culminating with the predominance of escape Of the bacillus and intracellular destruction (Figure 4).
The presence of these mediators of the inflammatory reaction and the predominant cell pattern in the Lepra skin lesions were analyzed by immunohistochemical methods, being observed in a proportion (2: 1) of T4: T8 lymphocytes in HT and HV lesions (0.5-1), a different arrangement of the inflammatory infiltrate cells for the two forms of the disease. Another factor that may favor the maintenance of suppression is the presence of the cytokine TGF beta, is present in marked amounts in HV lesions and the interpolar forms in decreasing levels of HDV and HDT, being absent in HT lesions. Additionally it was found in the inflammatory infiltrate of HV lesions absence of immunoreactivity to TNF alpha and depression of detection of nitric oxide, determined by the enzyme Nitric Oxide Synthetase induced I-NOS.
In contrast, excerpting cellular immunity and the production of pro-inflammatory cytokines (IL-1 and TNF-alpha) as well as in HT, contain bacillary proliferation, and prevent its spread, which may become harmful to the organism and cause Skin or nerve damage, due to the absence of regulatory factors.
TGF beta stimulates maturation and proliferation of suppressor T lymphocytes. As in the HV depression of the cytotoxic activity was described, the sum of these factors that favor the HV immune depression could be related to TGF beta.8 TGF beta inhibits the release of bactericidal mechanisms (ROI and RNI) in antagonistic action to gamma INF, during macrophagic activation. Inflamma induces bacillary destruction, reduces the viability of M. leprae, increases the frequency of EN outbreaks, and stimulates the production of TNF alpha by mononuclear cells. In HV this action is impaired by the presence of TGF beta, IL-4 and IL-10.
In serum and cultures, measurements of TNF alpha, IL-1 and IL-6 were depressed in virchowians, associated with marked amounts of IL-4 and TGF-beta in cutaneous lesions. This picture of immunological depression of Virchowians can be interrupted by abrupt episodes of alteration of the immune response, as in the EN type reaction. The severity of these states is associated with elevated levels of TNF alpha cytokines, suggesting that TNF alpha associated with high concentrations of alpha INF are important in the development of the complex symptomatology of erythema nodosum, favoring bacillary destruction. But for that to happen, concomitant chemotherapy is required. Therefore it can be assumed that during partial reaction or partial recovery of the microbicidal capacity potentiated by multidrug therapy (Figure 5).
Leprosy due to the wide spectrum of clinical presentation and the commitment of innumerable organs is characterized as a multidisciplinary disease that particularly involves the dermatologist because of the exuberant cutaneous manifestation. The polymorphism of lesions in the different clinical forms causes a series of differential diagnoses, making leprosy very attractive to dermatology. Every patient should be carefully examined through dermatological examination, sensitivity alteration test, bacilloscopy and biopsy, as the respective histopathological examination for classification of the clinical form and the therapeutic establishment.
The polychemotherapy treatment is based on periodic (monthly) controls for a maximum period of two years for the multibacillary form (DD, DV and HV). As the tolerance is very good and the adverse effects are rare, there is no need for laboratory tests, preceding the MDT, so it is recommended, when possible, a hemogram, a co-parasitology and liver enzymes. Confirming the diagnosis of the patient, the persons of his family and / or work, should be examined, if you will see if you received intradermal BCG vaccine. Two doses of BCG ID should be performed on relatives as a prophylactic measure. The therapeutic response is good.9 Treatment should be introduced as soon as possible, with the basic aim of reducing the development of disabilities and controlling endemic conditions in the country. Tables of neuritis, the main cause of disability, should be carefully evaluated and treated with corticosteroids in the acute phase. Now the reactions that occur as acute, can be controlled outpatient and tend to decrease in frequency and intensity during follow-up after discharge. What is important is to make the clinician aware that leprosy can be controlled as any chronic infectious disease, or that most of the symptoms that occur after discharge are associated with immunological reactions.
None.
None.

©2017 Jadue, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.