MOJ
eISSN: 2373-4442


Review Article Volume 5 Issue 3
1Servei d’Immunologia, Centre de Diagnòstic Biomèdic, Hospital Clínic de Barcelona, Spain
2Fundació Clínic per a la Recerca Biomèdica, Spain
3Institut d’Investigacions biomèdiques august Pi i Sunyer, Spain
4Plataforma d’Immunoterapia Hospital Sant Joan de Déu-Hospital Clínic de Barcelona, Spain
5Universitat de Barcelona, Spain
Correspondence: Manel Juan, Servei d’Immunologia Hospital Clínic C/ Villarroel, 17008036 Barcelona, Spain, , Tel 349-322-754-63 , Fax +349-345-180-38
Received: January 01, 1971 | Published: March 17, 2017
Citation: Marta ER, Arturo L, Berta M, Manel J, Maria C, et al. (2017) Future of Chimeric Antigen Receptors (Cars): Could it Drive Solutions Beyond Cancer? Examples in Autoimmune Diseases. MOJ Immunol 5(3): 00158. DOI: 10.15406/moji.2017.05.00158
CAR, chimeric antigen receptors; scFv, single-chain variable fragment; TCR, t-cell receptor; TRUKCs, t-cells redirected for universal cytokine-mediated killing; DCAR, dual chimeric antigen receptor; ICAR, inhibitory chimeric antigen receptor; CAAR, chimeric auto-antibody receptor; Treg, regulatory t-cell; BAR, b-cell-targeting antibody receptor
Chimeric Antigen Receptors (CAR) is a new Immunotherapy treatment, demonstrating an unexpected potentiality as antitumor treatment; appeared in the last years, it appears to be an effective option with great impact on the treatment of advanced refractory B-cell lymphoproliferative disorders (leukaemias and lymphomas, and even some myelomas). This novel therapy is a gene therapy vector-based treatment, by which this CAR that recognize a tumour antigen (such as CD19 for B-cell disorders) is designed in a vector (usually a lenti-o retrovirus) and introduced in the T lymphocytes of the patient; hence the name CART (chimeric antigen receptor T-cell), where these transduced T-cells can target those cells expressing those tumour antigens.1
The process to obtain a CART begins in the molecular design of the chimeric receptor by molecular engineering of the sequence, which will be introduced into T-cells (usually through a viral vector). After collecting T-cells usually from patient’s blood (leukoapheresis), they are ex-vivo modified by introducing CAR and by inducing its expression on the T-cell surface. After expanding these CAR+ T-cells, these cells are infused in the bloodstream of the patient to generate a specific immune response against tumour. CART cells become able to recognize cancerous cells through the specificity of a scFv (single-chain variable fragment) to detect an antigen on the surface of the tumour cells, and after that to kill them.2,3 The affinity and the avidity of the interaction between a CAR and its ligand are higher than the interaction between T cell receptor (TCR) and its peptide-MHC ligand; however, a CAR (instead of TCRs) is unable to recognize intracellular molecules.4 CAR consists of the combination of a scFv sequence, a transmembrane region and (usually at least two) domains activating for T-cells, such as signalling domains of CD3, CD28 and/or CD137.5 Although most of clinical trials are focused on refractory B cell lymphoprolipherative disorders, the CAR strategy is also being relied upon solid tumours. In any case the employment of CARs is not restricted to cancer therapy. A variety of CARs have been used to treat different pathologies such as viral or fungal infections.6 and autoimmune diseases, the main aspect reviewed in this article.
Solid tumours present several additional hurdles to the development of an effective CART immunotherapy5,7 and the use of this strategy is unlikely to be straightforward:8 main problems related with the failure in most of the trials are (a) the absence of a specific tumour antigen and the consequent on-target/off-tumour toxicity, (b) the inefficient homing of T-cells to tumour sites, (c) the short persistence of CAR-T cells, and (d) the immunosuppressive microenvironment in most of the tumours. These factors can considerably decrease the effectiveness of CART cells reducing their function. Furthermore, some clinical risk has to be taken into account. Probably the main achievement is to solve the on-target/off-tumour toxicity increasing the specificity and the affinity of the scFV.
Another problems associated to CARTs are
To overcome safety concerning caused by the lack of tumour-associated antigens many strategies have been proposed. Basically these alternatives consist on being able to distinguish between tumoral and normal cells, thus, reducing their side effects. Apart from the new generation of CARTs and TRUKCs (T cells redirected for universal cytokine-mediated killing)10 this new approaches have been developed by introducing:
Promising data from trials investigating T/NK-cells modified to express CARs against tumour antigens have generated extensive interest from clinicians, researchers and pharmaceutical companies, leading to broaden the applications of CARs beyond cancer through other pathologies, as autoimmune diseases or allograft rejection.13,14. Autoimmunity can be broadly separated in two processes: via self-reactive antibodies or “autoantibodies” (produced by plasma cells from the B lymphocyte lineage), and/or via self-reactive T-lymphocytes. This may occur during the processes of selection of those cells during their development (central tolerance), in which negative selection to self-antigens fails, or due to changes in target tissues (break of peripheral tolerance). Between these peripheral autoimmune mechanisms, there are the autorreactivitiy against the so-called “sequestered antigens” (self-antigens present in isolated areas of the organism that are not exposed in normal conditions), that become exposed after an infection or lesion, as it has been seen in multiple sclerosis,15 and also the mechanism named “molecular mimicry”16,17 where cross-reactivity of antigen receptors can generate an autoimmune response. Other proposed mechanisms for peripheral autoimmunity include anomalous expression of HLA in target cells, cytokine imbalance enhancing immune response, reducing regulatory mechanisms, or even intrinsic T regulatory cells defects.
Traditionally, the treatment for autoimmune diseases would consist in immune-modulatory/immune-suppressor drugs that act by a general reduction of the immune response. These approaches present problems in long term and high dose treatments, in which their suppressor effects can leave the patient vulnerable to infection and malignancy, next to some direct toxicity of the drug that can have a severe impact in the patient. Recently, new immunomodulatory and immunosuppressant agents have been developed, mainly focusing on trying to block not the whole Immune System, but more localized targets or pathways (so the secondary immunodeficiency generated is less severe), and also on lowering the toxicity and side effects, being better for long term treatments.18
The appearance of targeted monoclonal antibodies (such as anti-TNFα, anti-CD19 or anti-IL6R) has been an important leap forward for converting these “targeted treatments” into standards; due to the fact monoclonal antibodies interacts specifically with their targeted antigen, they still can cause several moderate states of immunodeficiency. An additional limitation can be also pointed out: as traditional treatment most of them don’t aim to restore the immune tolerance permanently, thus requiring continuous or long-term treatments. It is in these last points in which chimeric antigen receptor T-cell therapy and others could be a better option, as it provides a targeted tool, that could show less secondary effects, and aims to achieve a permanent restoration of the misbalanced Immune System.
Probably the most interesting approach for treating autoimmune diseases by CAR technology derives for a clever proposal for pemphigus vulgaris treatment: a chimeric auto-antibody receptor (CAAR) expressing Dsg3 on T-cells will only target against autoimmune B-cells expressing anti-Desmoglein 3 (Dsg3) surface immunoglobulin as specific receptor (Figure 1A&1B).19 This therapy proposes promising benefits: a decrease of Dsg3 auto-antibodies, specific cytolysis of autoreactive B-cells, and persistence of CAAR T-cells (at least detectable along 3 weeks, although it could be permanent if the autoantibody remains) and no off-target toxicity against other tissues. Thereby, CAARs seems to be an interesting strategy for autoantibody-mediated diseases (Figure 1B). More recently, two studies employed CARs to establish Factor VIII (FVIII) immune tolerance in haemophilia-A patients.20,21 In one of them (similar to CAARs, although anti-FVIII is not an autoantige), they created a similar structure to CAARs, named “BAR” (B-cell-targeting antibody receptor), transducing human Tregs with a BAR containing FVIII C2 domain or FVIII A2 domain, targeting FVIII-specific B-cells and deleting anti-FVIII antibody production. In the second study, a CAR contained a scFv that recognizes A2 domain of FVIII, called ANS8 CAR, was able to inhibit T and B cell responses to FVIII both in vivo and in vitro. The suppressive effect of this ANS8 CAR remained 8 weeks, whilst a re-challenge with FVIII was ineffective.
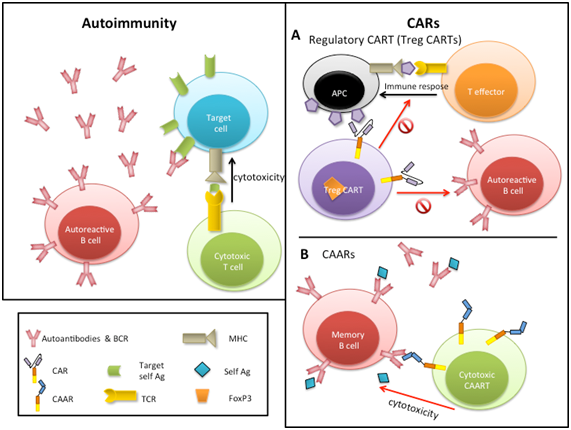
Figure 1 Autoimmunity mechanism and CAR therapies for autoimmune diseases. At left main elements involved in autoimmunity. At right.
(A) Treg CARTs and
(B) CAAR, chimeric auto-antibody receptor T cells.
As autoimmune diseases are characterized by a loss of tolerance, the use of cells with immunosuppressive functions like Tregs seems to be also a good alternative to restore the balance of the immune system. Pre-clinical studies had evidenced that adoptive transfer of Tregs can inhibit autoimmune response, resulting in a improvement of clinical symptoms in a variety of disorders such as autoimmune hepatitis, inflammatory bowel disease, systemic lupus erytematosus, autoimmune encephalomyelitis or arthritis.22 Antigen-specific Tregs have demonstrated to be more effective than polyclonal Tregs suppressing immune response, besides the lack of Tregs specificity may result in a generalized immunosuppression.23 However, the use of specific Tregs involves several difficulties, since they are at very low levels in a patient and consequently to reach appropriate levels to be infused into patients becomes a challenging task. Additionally, Tregs could change their immunosuppressive phenotype post-treatment, transforming into effector cells and exacerbating disease symptoms. Different strategies have been developed to solve these technical problems. Tregs encoding an anti-TNP antibody-based chimeric receptor have showed antigen-specific alleviation of acute experimental colitis in mouse models,24 opening the way for its use in inflammatory bowel disease. In a different study, Tregs were engineered to co-express FoxP3 and a CAR targeting myelin oligodendrocyte glycoprotein (MOG),25 achieving 2 objectives at once: maintenance of the suppressive state of Treg (avoiding their conversion into effector cells) and localizing the Tregs in the CNS (Figure 1A). Using a murine experimental autoimmune encephalomyelitis (a model of multiple sclerosis) and transnasal administration of CARαMOG Tregs, the study demonstrated that CARαMOG Tregs were capable of avoiding attacks against oligodendrocytes and diminishing symptoms of MS.
Although further studies are needed to support these novel CAR applications, this approach has shown promising results to specifically regulate adverse autoimmune responses and although by now these proposals still show very preliminary results, they should be yet considered as options for treatment of autoimmune diseases.
The present study showed the pharmacological potential of the ethanolic extract of Neem bark. Our findings demonstrated that the F-EtOAc, obtained after saponification of EtCNeem, showed to be rich in phenolic and flavonoid compounds with antioxidant potential, as well as a nontoxic.
None.
The authors declare no conflicts of interest.
Authores were supported in their CAR works by projects ARI (Assistència i Recerca Intensiva), PI13/00676, PICI14/00122 and PIE/00033, integrated in the Plan Nacional de I+D+I and cofinanced by the ISCIII-Subdirección General de Evaluación yFormento de la Investigación Sanitaria-and the Fondo Europeo de Desarrollo Regional.

©2017 Marta, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.