MOJ
eISSN: 2373-4442


Review Article Volume 4 Issue 1
1Immunology Service, Hospital Clínic-Centre de Diagnòstic Biomèdic Institut d’Investigació Biomèdica August Pi I Sunyer (IDIBAPS), Spain
2Immunoallergy Unit, Hospital Sant Joan de Deu, Spain
3Functional Unit of Immunology, SJD-Clinic, USA
4Universitat de Barcelona, Spain
Correspondence: Manel Juan, Servei d’Immunologia, c/ Villarroel 170, 08036 Barcelona, Spain,
Received: August 04, 2016 | Published: September 6, 2016
Citation: Arturo L, Marta E, Anna MV, Ana E, Ruiz et al. (2016) From Primary Immunodeficiency to Autoimmunity: How Extreme Situations Highlight the Main Genetic Factors Involved in Autoimmune Disease. MOJ Immunol 4(1): 00113. DOI: 10.15406/moji.2016.04.00113
Autoimmune diseases are a broad group of disorders characterized by the involvement of adaptive immunity but also the innate immunity. Genetic defects underlying autoimmunity are rare, though increasingly described, and represent excellent opportunities to get insight into the function of specific molecules. In this sense, primary immunodeficiencys are perfect examples for increasing knowledge of how the immune system works. Although most of immunodeficiencies manifest mainly susceptibility to infections, autoimmunity is probably the second group of clinical signs. Both adaptive and innate immunodeficiencies are being broadly related with autoimmune disorders. This article reviews the main aspects regarding autoimmunity learned from immunodeficiencies; from dysregulation of classical humoral and cellular immunodeficiencies to dysregulation of innate immunodeficiencies, which are associated to auto inflammatory disorders.
Keywords: genetics, autoimmunity, primary immunodeficiencies, UPR, autoinflammatory diseases, HLA, inflammatory disorders, IS
IS, immune system; APDS, activated PI3KD syndrome; CTLA-4, cytotoxic T-lymphocyte protein 4; LRBA, lipopolysaccharide-responsive beige-like anchor; CVID, common variable immunodeficiency; ICOS, inductible costimulatory molecule; CMC, chronic mucocutaneous candidiasis; STAT3, signal transducer and activator of transcription 3; ER, endoplasmic reticulum; AGS, aicardi-goutières syndrome; UPR, unfolded protein response; IFNB1, increasing the transcription of the type 1 interferon gene
Autoimmune diseases are a broad group of disorders characterized by the involvement of specific adaptive immunity as well as innate immunity. Although environmental factors such as stress factors inducing changes In hormone level, infectious injuries and specially own microbiota are also entailed in variable degrees,1 genetic factors have been broadly defined along decades and have shown to play a clear key role in autoimmunity.2,3 Beyond polymorphisms of HLA (the main genetic factor identified among autoimmune diseases),4,5 many other genes (costimulatory molecules, cytokines and their receptors, signaling pathways, transcription factors, etc.) are being involved in autoimmunity by genome-wide association studies (GWAS). But variability of this implication is very high and in some cases the involvement of these genes are weak and not very clear.
The immune system (IS) has typically been divided into innate and adaptive responses according to the cell types or molecules involved in eliminating pathogens. Both impairment and/or hyperactivation modifying either the innate or the adaptive immune responses may result in disease. For example, a decreased activation of innate or adaptive immune response may result in immunodeficiency, which is mainly characterized by increased susceptibility to infections.6
Although most of primary immunodeficiencies (PIDs) manifest mainly this susceptibility to infections, autoimmunity probably is the second group of features detectable in these patients. An exaggerated activation of the adaptive IS leads to the generation of self-reactive T and/or B-lymphocytes with high-titer autoantibodies defining autoimmunity.6 In contrast, the hyperactivation of the innate IS in the absence of microbial infection or autoantibody production give rise to autoinflammatory diseases.7 The boundaries between these three entities (immunodeficiency, autoimmunity and autoinflammation) are in fact fluid, and in many conditions the clinical features may overlap or can be a consequence of it. This review exposes and discusses main elements and concepts of mains relations between these three groups of immune disorders: primary immunodeficiencies, autoimmune and autoinflammatory diseases.
Genetic epidemiological studies have long suggested that many autoimmune diseases have an inherited component that may interact with environmental factors to determine the disease development. Autoimmunity is often described as having polygenic or complex inheritance. The five best-characterized monogenic autoimmune disorders classified as PIDs8 and discussed in this review are humoral immunodeficiencies, Autoimmune Polyendocrinopathy with Candidiasis and Ectodermal Dystrophy (APECED), X-linked Immunoproliferative Enteropathy (IPEX), Autoimmune Lymphoproliferative Syndrome (ALPS) and recently, haploinsufficiency of cytotoxic T-lymphocyte protein 4 (CTLA-4). Also hematological cytopenias can be detected in several PIDs: CD27 deficiency, Lipopolysaccharide-Responsive Beige-like Anchor (LRBA) deficiency, Activated PI3KD Syndrome (APDS) involving PI3KR1 and PIK3CD genes, X-linked immunodeficiency with magnesium defect (MAGT1 deficiency), and others.9 All these genes are distributed all along the human chromosomes (Figure 1). Autoimmune mechanisms can be also detected in these PID, although autoimmunity is expressed through similar mechanisms to humoral immunodeficiencies (where most of these PID were included until very recently) and CTLA-4 defects.
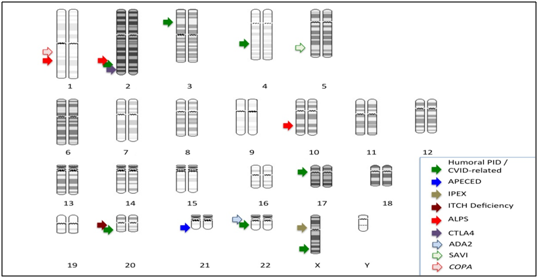
Figure 1 Chromosomal distribution of main genes of primary immunodeficiencies associated with autoimmunity. Arrows signal positions of main groups and genes.
CVID: TNFRSF13B, 17p11.2; TNFRSF13C, 22q13.1-q13.31; ICOS, 2q33; LRBA, 4q31.3; TNFRSF5, 20q12-q13.2; TNFSF5, Xq26; PRKCD, 3p21.31; APECED: AIRE, 21q22.3; IPEX: FOXP3, Xp11.23; ITCH Deficiency: ITCH, 20q11.22; ALPS: FAS/ TNFRSF6 , 10q24.1; FASL, 1q23; CASP10, 2q33-q34; CTLA4: CTLA4, 2q33; ADA2: CECR1, 22q11.2; SAVI: TMEM173, 5q31.2; COPA: COPA, 1q23.2
The arrival of next-generation sequencing technologies into clinics has unveiled new mechanistic pathways that lead to the discovery of novel autoinflammatory phenotypes that lead to excessive innate immune responses and overlapping adaptive immune dysfunction.10 These are the deficiency of adenosine deaminase 2 (DADA2) and STING-associated vasculopathy with onset in infancy (SAVI) that share characteristics with autoimmune disease and will be also reviewed in this article. Deciphering the underlying genetic cause of a suspected autoinflammatory disease may help to understand the molecular pathways that drive clinical phenotypes, some aspects of inflammation in autoimmunity and consequently to inspire further research for novel treatment targets looking forward the best therapeutic treatment option.
In fact, genetic defects are fortunately very rare situations, but these “extreme” presentations are excellent opportunities to learn about how molecules really work. Both PID that involves molecules of adaptive and innate IS are being broadly related with autoimmune disorders. This article wants to review main lessons obtained from PIDs: from dysregulations of adaptive IS linked with classical immunodeficiencies to dysregulation of innate IS, some of them defining autoinflammatory disorders.
Environmental factors, infectious experiences and the microbiome through their molecular mimicry between antigenic peptides and autoantigens should be also taken into consideration to understand how immunoreceptors finally react with usually normal tissues. Moreover, also stressors and other behavioural factors could cause a cortisol-mediated reduction of immune response or a more general induction of autoimmune responses and subsequent pathologies (Hashimoto thyroiditis, Rheumatoid arthritis, Psoriasis, etc).11
Common Variable Immunodeficiency (CVID) is the most frequent symptomatic PID in adults. Onset tends to occur between the 2nd to 3rd decades of life, although it can be diagnosed from childhood to old age. It is mainly characterized by the presence of low immunoglobulin levels (at least 2 standard deviation below the mean for age in serum IgG and IgA) and a dysfunctional response to specific antibodies (ESID-PAGID criteria “probable CVID”, www.esid.org). T CD4+ cytopenia and decreased lymphocyte proliferative response to mitogens are also common, and can be associated to recurrent respiratory tract infections caused by the typical susceptibility to infections. Risk of neoplastic development is also higher in those patients (specially lymphoma and GI carcinoma). Among this variable group of possible presentations, we want to remark that CVID is linked to a plethora of autoimmune conditions, such as idiopathic thrombocytopenia purpura, autoimmune hemolytic anemia, granulomatous lung disease, inflammatory bowel disease and autoimmune thyroiditis.12
Genetic studies of CVID patients have led to believe that although there is a common phenotype among patients, genetic factors are heterogeneous.13 Many specific monogenic mutations have been identified, such as transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI) deficiency, caused by mutations in the TNFRSF13B gene, which causes a defect in the binding with BAFF and APRIL, a decrease in expression of BLIMP-1, and therefore affecting B cell maduration and T-cell-independent class-switch recombination in B cells.14 Deficiencies in BAFF-R have also been reported, with similar outcome.15 Deficiency of the inductible costimulatory molecule (ICOS) also causes an impaired class-switch in B cells affecting the interaction between B and T cells, with patients displaying autoimmune conditions,16 with higher IL-17 levels and impaired IL-10 secretion.17 Lipopolysaccharide responsible beige-like protein (LRBA), involved in vesicle displacement, and directly linked to CTLA4, when deficient exhibits inflammatory bowel disease and other autoimmune diseases besides the hypogammaglobulinemia.18 Deficiency of CD40 and CD40L due to mutations in TNFRSF5 and TNFSF5 genes respectively, are also associated with autoimmunity, as also occurs in PRKC-δ deficiency, in which a mutation in the PRKCD gene provokes the failure in a protein kinase C protein that regulates immune cell survival, proliferation and apoptosis.9
Autoimmune-mediated cytopenias in PID include autoimmune thrombocytopenia (AITP), anemia and neutropenia.12 A correlation between the expansion of a subset of CD21low/dim B cells with splenomegaly has been found, which could be responsible of the cytopenia.19 This subset of cells is a group of anergic B cells with defective signaling, capable of homing to sites of inflammation.20 Also related to splenomegaly is the presence of low levels of class-switch memory B cells. Unexpectedly, near absent absolute numbers of plasmablasts in CVID have been associated to autoimmune cytopenias as well.21
Besides the direct relationship between B cell alterations and immune cytopenias, other cells, such as FOXP3+ T reg cells have shown an important role in suppressing these autoimmune processes.21 Reduced proportion of those FOXP3+ T cells have been reported in CVID patients with associated autoimmunity.22 Also, soluble BAFF/Blys, an important B cell proliferation factor, along with TACI and APRIL, appear to be elevated in some studies,23 a feature that has been already reported in the context of systemic autoimmune diseases (specially rheumatic diseases).
APECED is a rare autosomal recessive disorder of immune dysregulation characterized by chronic mucocutaneous candidiasis (CMC) and polyendocrinopathy (hypoparathyroidism, Addison's disease, ovarian failure, pernicious anemia, type I diabetes and hypothyroidism), and other features (tooth enamel hypoplasia, nail dystrophy and keratopathy). Patients do not appear to have increased susceptibility to other types of infection besides candidiasis. Onset occurs typically during childhood, and it is diagnosed when the presence of two of the three classical components are identified: CMC, chronic hypoparathyroidism, and/or Addison's disease.24
APECED is caused by mutations appearing in the Autoimmune Regulator (AIRE) gene. Located in the 22q22 region, the gene consists of 14 exons spanning 11.9 kb of genomic DNA and encodes for a 545 amino acid protein with a molecular weight of 58 kDa working as a “non-classical” transcriptional factor in immune related organs.25 Its highest expression occurs at the medullary thymic epithelial cells (mTEC), and at secondary lymphoid organs (lymph nodes, fetal liver, and spleen). In the thymus, this induces ectopic expression of self-antigens that are otherwise restricted to peripheral tissues, the tissue-restricted antigens (TRA). If this is carried either acting directly as a transcription factor or by regulating mTEC maturation cycle still requires further studies. This expression of TRA in the mTEC cells allows for the negative selection of self-reactive T cells and the induction of central tolerance.
Most frequent mutations in AIRE genes usually show an autosomal recessive mode of inheritance, though dominant modes have also been reported.26 It shows a higher prevalence in genetically isolated populations, like Iranian Jews (1:9,000), Finish population (1:25,000), and Sardinian populations (1:14,400), and there is a tendency for certain mutations to be peculiar of the different populations.27 About 70 different AIRE gene mutations have been reported, including single nucleotide substitutions, small insertions, deletions and splicing alterations. These mutations can modify the distribution of the protein in the cell or render it non-functional.
A lack of AIRE gene expressions allows auto-reactive T cells to escape negative selection at the thymus, which can cause autoimmunity in the peripheral tissues and organs. Because of that, organ-specific T-cell mediated immunity has been widely studied in APECED patients and it has allowed to identify alterations in the regulatory T cell population such as decreased in CD4+CD25+ T cells,28 FOXP3+ T cells, and reduced levels of expression of FOXP3,29 which support the theory that this patients show a disbalance in the effector activity of the immune system against the regulator activity.
Considering the complexity of the molecule, it is possible that specific mutations would generate different functional abnormalities, and therefore, different phenotypic expression. CMC has been associated with specific autoantibodies against IL-22 and IL-17F.30 Regarding the innate IS, it has also been hinted that there might be a relation between AIRE protein and Dectin 1 in peripheral cells,31 which might cause a defective recognition of β-glucan and anti-Candida responses. Autoantibodies against IFN-α and IFN-ω have also been identified32 (they have been detected in almost all APECED patients, even at early stages, which could make them a useful diagnostic marker). Other specific clinical features associated with specific autoantibodies have been identified, such as NALP5 (hypoparathyroidism) and CYP21, CYP11A1 or CYP17 (Addison's disease).33 However, the efforts to detect specific autoantibodies associated to specific AIRE mutations so as to establish a genotype-phenotype correlation have failed, and there are reports of families carrying the same AIRE mutation with a wide variety in phenotypes.34
IPEX syndrome is a rare X-linked recessive disease characterized by intense diarrhea due to an autoimmune enteropathy (with villous atrophy), associated growing defects, atopic dermatitis, autoimmune diseases (diabetes, thyroid disease or autoimmune cytopenias), and increased serum levels of IgE and eosinofilia. It was first described in 1982 in a family with 19 affected males across five generations.35 Onset happens usually in the first year, and if not aggressively treated (with immunosuppression or bone marrow transplantation) it can be fatal in the span of one or two years due to metabolic failure or sepsis.
Some years after the initial clinical descriptions, mutations in the Forkhead box p3 (FOXP3) gene, in the centromeric region Xq11.3-q13.3, were identified in patients with the IPEX phenotype.36 Murine models (the so called “scurfy mouse”) and mutations in other IPEX patients confirmed the role of FOXP3in the disease.
FOXP3is a highly conserved gene, composed by 12 exons encoding a 431 amino acids protein in humans. More than 60 mutations have been reported, almost half of them in the C-terminal forkhead DNA-binding domain, and also 2 of them in the polyadenylation site of the gene, which causes an unstable FOXP3mRNA, leading to a severe, early onset of the disease.37 Those patients with a mutation that does not allow the expression of functional FOXP3protein often provokes a severe onset of the disease.38 The majority of individuals tend to have missense mutations that produce normal or reduced levels of the mutant protein, which lead to an alteration of the transcriptional regulatory activity (as the DNA-binding sites have been altered), the interactions with other molecules, or FOXP3dimerization.
The role of FOXP3is that of a master regulator in the thymic-derived regulatory T cells (nTreg), whose function is to maintain peripheral self-tolerance, among others. CD4+CD25+FOXP3+ T cells can be present in normal numbers in IPEX patients, which orientates to a functionally impaired protein (the functionality of which might determine the severity of the disease), although some studies show lower survival rates in FOXP3mutated Treg cells in the periphery.39,40 In addition to the failure of Treg function, mutated FOXP3may be responsible for a conversion of the Treg cells to IL-17-producers effector cells, thus potentiating inflammatory processes.
IPEX patients also exhibit an impaired Th1 related cytokine production in its peripheral cells, and there appears an accumulation of autoreactive mature naïve B cells in the peripheral blood, secondary to the Treg function failure.40 The type of autoantibodies produced by B cells in IPEX patients are diverse and includes anti-mitochondrial, anti-islet cell, anti-insulin, anti-GAD, anti-thyroid peroxidase, anti-thyroglobulin, anti-enterocyte, anti-platelet, or anti-neutrophil antibodies.41
There is not a strict genotype-phenotype correlation, as many subjects in a family with the same mutation exhibit a different clinical profile, this may be caused by the complexity of the interactions of FOXP3and also due to environmental or epigenetic factors.42
CD25 deficiency
Deficiency in CD25 or alpha chain of interleukin-2 receptor alpha (IL-2Rα) is associated with a phenotype that includes endocrinopathies, eczema, hemolytic anemia, lymphadenopathy, hepatosplenomegaly, enteropathy, and impaired T cell function, in a similar way to IPEX syndrome. IL-2Rα, in conjunction with the β-chain and γ-chain, defines high affinity IL-2R, the receptor for IL-2 (being alpha chain the most specific subunit for IL-2), a cytokine fundamental for expanding and defining function of T regulatory cells (Tregs) in the periphery.43,44 CD25 is constitutively expressed at the highest levels in these Tregs, allowing a fast response to IL-2 in the immune response, reducing immune function.45 It also promotes FOXP3transcription in a similar way to STAT5.46 One of the signaling molecules of IL2R.
Although IL-2Rα only contributes increasing IL-2 binding affinity, it has been reported that truncated IL-2Rα molecules due to a mutation produces an IPEX-like PID, which could explain some of the previously diagnosed IPEX-like syndromes that showed no detectable mutations in the FoxP3gene.47 Despite this fact, differencies must be considered in CD25 deficient patients and IPEX syndrome patients,48 such as intense inflammatory response to CMV, enteropathy, elevated IgE levels, and also impaired IL-10 production.49
STAT3 Gain of function
The transcription factor signal transducer and activator of transcription 3 (STAT3) is a transcription factor encoded by the gene of the same name. STAT3, when triggered by cytokines and growth factors (such as IFNs, EGF, IL5, IL6, HGF, LIF and BMP2) is phosphorylated by Janus kinases, forming homo or heterodimers able to translocate to the nucleus of the cell to act as activators of transcription, mediating the expression of other genes, related specially to cell proliferation, differentiation and survival, and inflammation processes.50
When mutations in STAT3 gene generate a loss of functions, a syndrome known as HyperImmunoglobulin E Syndrome (HISE) is manifested; this autosomal dominant disorder is characterized by high serum Ig E levels, dermatitis and recurrent lung infections. But when STAT3 mutations produce a gain of function of the molecule, the genetic disorder becomes characterized by early onset of multiple autoimmune organ-specific pathologies and autoimmune cytopenias, next to lymphoproliferation, susceptibility to infections, and, in some cases, also failure to thrive;51,52 both, inherited and de novo cases have been reported.53
Considering the important role of STAT3 in Th17 lymphocyte signalling by IL6, a possible way to explain the clinical features in STAT3 GOF comes from understanding that this gain of function leads to an increase of Th17 function54 and at least in some patients a lower levels of T regulatory cells by a decreased expression of FOXP3and/or CD25; all these elements could explain its phenotypic similarity to IPEX-like syndromes.53
TPP2 deficiency
Tripeptidyl-peptidase II (TPP2) is a molecule involved in extralysosomal peptide degradation capable to remove tripeptides from the N-terminal side of polypeptide chains, especially in some molecules related to antigen presentation by MHC class I.55 TTP2 has shown relevance in cell survivability and proliferation, contributing to an antiapoptotic phenotype.56 In murine models, TPP2 defect has shown evidence of premature senescence and proliferative apoptosis in T cells, also affecting fibroblasts and exhibiting systemic alterations, but with no autoimmunity processes.57
However patients with loss of function mutations in TPP2 have been reported with a phenotype of variable lymphoproliferation, severe autoimmune cytopenias, hypergammaglobulinemia, susceptibility to infections, B and T cells immunosensecence including other major phenotypic alterations associated to defects in apoptosis, proliferation and lymphocyte functions.58 It has been theorized that the defective TPP2 is unable to control senescence leading to this clinical and immunological features, akin to those that happen gradually in the normal aging of the immune system, but in a more “acute” process.59
ITCH deficiency
Human ITCH deficiency is a very rare disease the causes a combination of clinical features that include affected physical growth, distinctive craniofacial morphology, impaired muscle development, lung disease, which can be fatal and immune dysregulation, consisting in autoimmunity and impaired T cell function.60 In these patients, autoimmunity is mainly organ-specific, in liver, intestine and lung.
This disease has been described in groups of Old Order Amish, which presented the above mentioned symptoms and also organomegalia, that might be caused by the accumulation of non-degraded proteins in the target organs.60
ITCH gene encodes for a E3 ubiquitin ligase, a protein tasked with the degradation of proteins in the proteosome through ubiquitination, which has proven important for the immune system, playing a pivotal role in lymphoid cell differentiation and immune response regulation.61 Ubiquitination of the T cell receptor mediates its downregulation, and downstream signaling may affect the IL-2 production and the T cell proliferation.62 A defect in the E3 ligase, failing to perform the ubiquitin attachment, can cause an indiscriminate activation of T cells and the loss of self-tolerance. In murine models, ITCH deficiency has been proven to induce higher Th2 cytokine expression by Treg cells, although FOXP3+ cells and immune regulation were not affected.63
Autoimmune lymphoproliferative syndrome (ALPS)
Autoimmune lymphoproliferative syndrome (ALPS) is a hereditable disorder of apoptosis, resulting in accumulation of autoreactive lymphocytes.64 It usually presents in early childhood as nonmalignant lymphadenopathy, with hepatosplenomegaly and autoimmune multilineage cytopenias [65-67]. ALPS patients typically present with an increased double-negative T (DNT: T-cell receptor TCRαβ+CD3+CD4-CD8-) cells in peripheral blood and lymphoid tissues, diminished CD27+ memory B cells, defective lymphocyte apoptosis in vitro, autoantibodies, skewed T helper cell responses, including elevated levels of interleukin-10 (IL-10), soluble Fas-ligand and vitamin B12.64 Moreover, patients with mutations affecting the intracellular portion of FAS have an increased risk of developing Hodgkin and non-Hodgkin lymphoma.67
FAS (also called Apo-1, CD95 and TNFSF6) is a cell-surface receptor belonging to the tumor necrosis factor receptor (TNFR) superfamily. Upon binding to its ligand (FAS ligand), it undergoes conformational changes that allow the formation of the death-inducing signalling complex, consisting mainly of the FAS-associated death domain (FADD) and the caspase 8 and caspase 10 proteins, which initiates a cascade of events that culminate in cells’ apoptotic death. Most of patients with ALPS carry a germline mutation in the FAS gene. Heterozygous autosomal dominant mutations in FAS account for approximately 65% of all ALPS cases, and are classified as ALPS type Ia. Other cases of ALPS have been associated with mutations in the genes encoding FAS ligand (ALPS Ib), caspases 8 or 10 (ALPS type II), or NRAS (ALPS type IV), whereas cases without mutations are classified as ALPS type III or ALPS phenotype.68–71 Although ALPS type III patients meet all the defining criteria of ALPS; defective in vitro apoptosis, lymphadenopathy and splenomegaly, and increased DNT cells, they lack a demonstrable in vitro apoptosis defect to the FAS pathway. In fact, some of those ALPS phenotype patients classified previously in ALPS type III have been identified as having a somatic FAS mutation mainly in their DNT cell population.72 Therefore, it is highly recommended testing for somatic FAS mutations in all ALPS type III patients.73
FAS mutations have variable disease penetrance, with only 60% penetrance among family members harbouring the same heterozygous gene mutation.74 Some of the possible explanations of variable disease penetrance in ALPS have recently been suggested. On the one hand, Kuehn et al., showed that FAS happloinsufficiency caused by nonsense or frame shift mutations affecting the extracellular domain may be associated with low penetrance whereas mutations in the intracellular FADD may be associated with high penetrance.75 On the other hand, Magerus-Chatinet et al.76 observed that the acquisition of a somatic mutation in the second FAS allele in patients with an already inherited heterozygous FAS mutation, contributed to the typical clinical manifestations of ALPS, whereas patients who only carry the heterozygous FAS mutation remained asymptomatic.76 In contrast to these reports, other authors suggested that penetrance is not related to the type of mutation and is probably determined by secondary genetic and environmental modifiers.77
Heterozygous germline mutations in cytotoxic T-lymphocyte protein 4 (CTLA-4)
CTLA-4 (also known as CD152) is an inhibitory receptor expressed on the surface of activated T cells and constitutively expressed in regulatory T cells. Upon binding its ligand (B7 molecules expressed on antigen-presenting cells) it becomes activated and inhibits the proliferation of effector T cells and stimulates the suppression functions of Treg. Thus, CTLA-4 has a crucial role in maintaining tolerance to self-antigens. Several cancer treatments based on CTLA-4 blocking antibodies have demonstrated its powerful role in regulating lymphocyte homeostasis.78
In mice, homozygous CTLA-4 mutations results in a lethal autoimmune phenotype, whereas heterozygous mice do not show a detectable phenotype.79 Recently, Schubert et al.80 identified a large family with 5 family members affected from severe autoimmunity which have a heterozygous CTLA-4 mutation.80 The family members were previously diagnosed with CVID or selective IgA deficiency. They presented with recurrent respiratory tract infections, hypogammaglobulinemia, autoimmune cytopenia, autoimmune enteropathy and granulomatous infiltrative lung disease. In addition, they present an extensive CD4+ T cell infiltration in a number of organs including the intestines, lungs, bone marrow, central nervous system and kidneys.80 Moreover, screening of other unrelated patients with comparable clinical phenotypes identified additional families with previously undescribed mutations in CTLA-4.81 Clinical penetrance was incomplete, as some heterozygous individuals were asymptomatic. This incomplete penetrance in disease may account for CTLA-4 happloinsufficiency, as already shown in ALPS and it may result from a combination of genetic, environmental, and lifestyle factors, many of which are unknown.
Deficiency of adenosine deaminase 2 (DADA2)
In 2014, two independent groups identified a new vascular inflammatory syndrome caused by loss-of-function mutations in CECR1 (cat eye syndrome chromosome region, candidate 1) gene, which encodes for the adenosine deaminase 2 (ADA2).82,83 Genetic susceptibility for the development of systemic vasculitis has been previously suspected and confirmed in genome-wide association studies.84 However, these are the first reports in which is postulated a recessive mode for disease inheritance in a subtype of systemic necrotizing vasculitis previously considered idiopathic.
This inflammatory vasculopathy is characterized by highly varied age at onset, severity and organ involvement, even within patients from the same families. Clinical manifestations range from early-onset recurrent strokes or fatal systemic vasculitis affecting medium and small-sized muscular arteries, classically resembling polyarteritis nodosa (PAN), to limited cutaneous manifestations in middle-aged persons. The disease evolution may thus be influenced by environmental factors which may interact with genetic factors to determine the course of the disease. Other clinical features described so far are livedo reticularis, recurrent fever, hepatosplenomegaly, arterial hypertension, ophthalmologic manifestations, myalgia, leg ulcers, Raynaud phenomenon, subcutaneous nodules, purpura, and digital necrosis.83 With regard to the laboratory parameters, antinuclear antibodies (ANAs) were positive in 3/24 and in 3/9 patients reported by Navon Elkan et al.83 and Zhou et al.82 respectively. At the onset of strokes, all patients described by Zhou et al. were negative for antiphospholipid antibodies whereas over time, lupus anticoagulant developed in 4 patients.
ADA2 has partial structural homology with human ADA1. Both proteins convert adenosine to inosine and 2’-deoxyadenosine to 2’-deoxyinosine. However, the affinity of ADA2 for adenosine is lower than that of ADA1. Whereas ADA1 is monomeric and largely intracellular, ADA2 is a dimeric and secreted protein. Inherited ADA1 mutations result in severe combined immunodeficiency disease (SCID) with a defect in T and B-lymphocytes. By contrast, patients with ADA2 deficiency have only a mild immunodeficiency mostly restricted to B cells. ADA2 is known to be produced by myeloid cells and to promote macrophage differentiation. One of the key mechanisms proposed for the pathogenesis of DADA2 is that monocytes promptly differentiate into M1 (pro-inflammatory) macrophages, but few differentiate into M2 (anti-inflammatory) macrophages, thus giving rise to vasculopathy and inflammation. It has also been postulated that activating neutrophils may also contribute to the inflammatory process.85 The measurement of ADA2 activity in plasma can be used as a diagnostic test for establishing the pathogenicity of novel CECR1mutations since patients with DADA2 had significantly diminished plasmatic ADA2 activity. To date, 16 different pathogenic mutations have been described (available in INFEVERS database, http://fmf.igh.cnrs.fr/ISSAID/infevers/). Different therapeutic approaches are being currently considered, ranging from anti-TNF agents, ADA2-replacement therapy with periodical infusions of fresh-frozen plasma or recombinant ADA2, and allogeneic hematopoietic stem cell transplantation (HSCT).82,86
STING-associated vasculopathy with onset in infancy (SAVI)
Type I interferon (IFN) modulate both innate and adaptive immune responses against intracellular invaders. Dysregulation of either IFN signalling or its production can lead to inflammatory disease, including Aicardi-Goutières syndrome (AGS), autoimmune diseases such as systemic lupus erythematous (SLE), and a growing number of conditions that clinically present as autoinflammatory diseases.87
Aicardi-Goutières syndrome (AGS) is an autosomal recessive inflammatory disease particularly affecting the brain and skin, occurring as a result of mutations in a total of six genes that lead to an increased interferon signalling and define six different phenotypes of AGS (AGS1-AGS6).87–90 Recently, gain-of-function mutations in IFIH1 gene (also known as MDA5), encoding a cytosolic viral RNA receptor, were identified in patients with a spectrum of severe neurological impairment and immunological disease, including classical AGS.91 It has been described that most individuals with the autoimmune disease SLE demonstrate an interferon signature92,93 and polymorphisms in IFIH1confer increased risk of developing SLE.94
Similarly, the recent identification of autosomal dominant mutations in TMEM173, the gene encoding for the stimulator of interferon genes (STING) has allowed the description of a new autoinflammatory syndrome called STING-associated vasculopathy with onset in infancy (SAVI).95 It is characterized by neonatal-onset systemic inflammation, a severe cutaneous vasculopathy leading to extensive tissue loss, and pulmonary inflammation, which in some cases can progress to fatal interstitial lung disease. In contrast to AGS, CNS disease is not typically seen in SAVI. Autoantibody production was common but titers were transiently positive in the sporadic patients reported by Liu Y et al.97 and thereby they were not apparently associated with disease severity. Surprisingly, a recent report contradicts the aforementioned statement and describes a familiar case of an inherited STING-activating mutation with a variable clinical symptoms of systemic inflammatory syndrome and lupus-like manifestations which in that case, the autoantibody titers correlates with a more severe disease course.96
STING is an endoplasmic reticulum transmembrane protein, first described in 2008 as being essential in the transduction of the type 1 interferon pathway. STING is activated upon binding of cyclic guanosine monophosphate-adenosine monophosphate (cGAMP) that is synthesized by the enzyme cGAS, a cytosolic DNA sensor, which is activated upon binding double-stranded DNA (dsDNA). The STING mutations identified in SAVI patients are all closely clustered at or near the critical region for protein dimerization. All of them are gain-of-function mutations leading to a constitutively activated STING that in turn, provokes a chronic overproduction of IFN-β by means of increasing the transcription of the type 1 interferon gene (IFNB1). The binding of IFN-β to its receptor activates Janus kinases (JAKs), including JAK1 and tyrosine kinase 2 (TYK2), which in turn phosphorylate the receptor. This process allows the binding of STAT1 and STAT2 to the receptor, their phosporylation, dimerization and finally translocation of the dimer to the nucleus where it up-regulates the IFNB1gene transcription generating a positive feedback loop that further up-regulates STING and other proinflammatory cytokines.
All SAVI patients show persistently high IFN signatures in the blood resembling other interferonopathies including the proteasome defect-associated autoinflammatory disease CANDLE,97 the AGS and TREX1-mediated familial childblain lupus.98 Thus, it clearly provide new evidence that different defects in the interferon pathways may result in overlapping clinical features among the spectrum of the monogenic autoinflammatory diseases at one end to monogenic autoimmune conditions at the other.
Liu and colleagues demonstrate that, patients cells treated in vitro with oral JAK inhibitors showed suppression of STAT1 phosphorylation and a reduction of IFN-β production. Therefore, it suggests that blocking IFN signalling with the JAK inhibitor baricitinib may offer a therapeutic strategy not only for SAVI, AGS and CANDLE but also for other interferonopathies such as SLE.95
Hereditary autoimmune-mediated lung disease and arthritis.
Defects in the intracellular protein trafficking lead as a result, endoplasmic reticulum (ER) stress and activation of unfolded protein response (UPR), both of which have been strongly associated with the pathogenesis of inflammatory and autoimmune disorders.99 Recently, Watkin et al.100 described a new mendelian syndrome of autoimmunity in five unrelated families characterized by high-titter autoantibodies, interstitial lung disease and inflammatory arthritis.100 It has an autosomal dominant mode of inheritance with an incomplete penetrance and is caused by heterozygous mutations in COPA gene, which encodes the COPA subunit of COPI. COPI and COPII are carrier complexes required for membrane trafficking between the ER and the Golgi. The research group demonstrates by dynamic functional experiments that mutant COPA is unable to bind correctly to other proteins and leads to a defective intracellular trafficking. Further studies of Watkin et al.100 showed that the expression of mutant COPA in cells induces higher levels of ER stress that lead to a cytokine milieu which promotes the generation of TH17 cells, a well recognized cell population that is implicated in autoimmunity.101,102
In this Review, we provide an overview of the main PID that present with autoimmunity and provide insights on how single-gene defects drive autoimmunity through both innate and adaptive immune response. We know that HLA loci as well as many genetic and environmental susceptibility loci are playing a crucial role in triggering disease and determining disease development. However, learning from well-defined monogenic heritable disorders such as PIDs is contributing to understand better the mechanistic pathways that drive autoimmune disease. Next-generation sequencing technologies are crucial for the elucidation of this genetic defects leading to autoimmunity. Future research on genetics underlying autoimmunity might bring us towards to a closer personalized medicine.
Some works related with this review were supported by the “Instituto de Salud Carlos III (ISCII) and “Fondo Europeo de Desarrollo Regional (FEDER)” (PI13/00676, PIE13/00033) from Spanish “Ministerio de Sanidad”, and grants “Premi Emili Letang” (A, Mensa-Vilaró) and “Ajut Josep Font” (EA González-Navarro) from “Hospital Clínic de Barcelona”.
The authors declare there is no conflict of interests.
None.

©2016 Arturo, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.