MOJ
eISSN: 2373-4442


Short Communication Volume 5 Issue 4
1Institute of Cell Biology, Faculty of Medical Sciences, National University of Cordoba, Argentina
2Institute of Hematology and Hemoterapy, National University of Cordoba, Argentina
Correspondence: Fernando Marcos Rodriguez, Institute of Cell Biology, Faculty of Medical Sciences, National University of Cordoba, Enrique Barros esquina Gordillo, Ciudad Universitaria, Cordoba, 5000, Argentina, , Tel 005-435-143-340-20
Received: January 01, 1971 | Published: April 21, 2017
Citation: Rodriguez FM, Vargas A, Miotti CC, Silva NG, Frattari SR, et al. (2017) Extracellular Traps and Co-Stimulatory Molecules in Leukocytes of Patients with Positive Serology for Chagas Disease DOI: 10.15406/moji.2017.05.00163
Extracellular traps (ETs) are composed of chromatin and intracellular proteins which are extruded in leukocytes in inflammatory conditions. We performed in vitro ETs generation and marking of costimulatory molecules in peripheral blood leukocytes taken from people with positive serology for Chagas. A higher number of ETs was found in samples with positive serology for Chagas, (p <0.0001). Expression of CD80 in neutrophils is shown in samples with positive serology for Chagas. The presence of costimulatory molecules could be related to the possibility of rupture of self-tolerance. Generating ETs could be related to the presence of antibodies and antigen-antibody complexes in serum of samples. Cytokines and inflammatory mediators would induce costimulatory molecules besides LPS stimulation. Further studies are needed to elucidate the molecular mechanisms that underlie the clinical evolution of patients.
Keywords: extracellular traps, costimulatory molecules, leukocytes, chagas disease
ETs, extracellular traps; LPS, lipopolysaccharide; NETs, neutrophil extracellular traps
Extracellular traps (ETs) are structures composed of chromatin, histones and granular proteins, first described on 2004 in neutrophils by Brinkmann et al.,1 constitute a new mechanism of defense of the immune system in response to diverse microorganisms and other varied stimuli. In stimulated cells, the development of this phenomenon begins with the decondensation of the chromatin simultaneously with the loss of the nuclear structure. As the nuclear sheaths are separated, cytoplasm and granules are shown unchanged. At a later time the nuclear membrane disintegrates into vesicles and the granules disintegrate, resulting in the mixing of the contents of the nuclear, cytoplasmic and granular compartments. Finally, the combination of all these components is thrown into the extracellular space.2 The most studied costimulatory pathway in T lymphocyte activation is the B7-1 (CD80)/B7-2 (CD86): CD28/CTLA4 pathway. Regulatory T lymphocytes are dependent for their generation and maintenance of costimulation by this B7: CD28 pathway.3 The co-stimulatory molecules CD80 and CD86 are constitutively expressed in some cell types and are classically described as molecules of professional antigen-presenting cells such as dendritic cells, macrophages and B lymphocytes. These molecules are expressed at low intensity in resting cells but can be induced by inflammatory cytokines and by stimulation of Toll-like receptors with microbial products such as lipopolysaccharide (LPS).3. In neutrophils, it has been observed that such costimulatory molecules can be stored in their cytoplasmic granules and under certain stimuli expressed on the cell surface.4 In a previous work, we have described the Colocalization of CD80 and CD86 costimulatory molecules in neutrophil extracellular traps (NETs) in cultures of total autologous leukocytes in healthy human blood samples.5
Chagas disease is a parasitic disease produced by the flagellate protozoan Trypanosoma cruzi transmitted to humans by the triatomines of the Reduviidae family.6 It affects about 10 million people in Latin America although due to migration, it has become a global health problem. In chronic stage, neomycovasculature has been described as an active factor in immunopathology.7 Bonney and Engman postulate if there are one or several mechanisms that influence such as parasite-induced immunity, primary neuronal damage, microvasculature damage, myocytolysis, antibody-mediated cytotoxicity, non-specific damage caused by eosinophils and neutrophils, among others.8.Pathologic inflammatory infiltrate in cardiac tissue with Chagas disease consists mainly of lymphocytes, but macrophages, plasma cells, eosinophils and neutrophils have also been observed.8 One hypothesis holds that late heart damage is initially due to the breakdown of self-tolerance.9 In the chronic stage of the disease, among other causes, the variability of immunogenic proteins that coexpress in the surface of the parasite results in the impossibility of totally eliminating T. cruzi by the host.10 In addition, some B-cell mitogens produced by the parasite contribute to polyclonal B-cell activation and hypogammaglobulinemia, which also delay the specific T. cruzi antibody response.10 On the other hand, as regards ETs, an in vitro study demonstrates that T. cruzi and soluble parasite antigens are able to generate NETs by stimulation of Toll-like receptors.11 In a previous study we observed the presence of spontaneous ETs in neutrophils with ring nuclei in total leukocyte cultures in patients with positive serology for Chagas' disease, without the need for LPS stimulation.12 Interestingly, Souza et al. demonstrated in an in vitro experiment that T. cruzi increases the frequency of CD80+ monocytes in asymptomatic patients and in patients with chronic chagasic cardiomyopathy; however CD86 decreases its expression in the latter group. Exposure of lymphocytes to T. cruzi-infected monocytes increases the expression of CTLA-4 in lymphocytes of asymptomatic patients, but not in those with chronic chagasic cardiopathy. These data suggest in asymptomatic patients that the parasite would induce an immunoregulation, given the increased expression of CD80 and CTLA-4.13
In the present work, we aimed to observe the occurrence of ETs, in human blood samples, in the context of serological positivity for Chagas' disease, as well as to perform labeling of costimulatory molecules. ETs generation: in samples with positive serology for Chagas' disease, a greater number of cells that spontaneously release ETs are observed. DAPI-stained ETs, morphologically distinguished as diffuse blue staining or as chromatin fibrils, were observed by means of Immunofluorescence Microscopy (Figure 1). The number of cells releasing ETs spontaneously was higher in culture samples with positive serology for Chagas disease, *** p <0.0001 (Student t for Independent samples). In addition, samples with positive serology for Chagas stimulated with LPS also showed a higher number of cells releasing ETs, compared to samples with negative serology stimulated with LPS, *** p <0.0001 (t of Student for independent samples) (Figure 2). Significant differences were observed between the number of cells releasing ETs in paired samples in all groups *** p <0.0001 (Student t for paired samples) (Figure 2). Immunofluorescence for CD80 and CD86: in samples with positive serology for Chagas disease stimulated with LPS the CD80 and CD86 molecules are expressed with normal distribution patterns, with some CD86 positive cells being observed. In addition, when samples were stimulated with LPS, neutrophils expressing CD80 were found and no CD86-positive cells were observed in samples with positive serology for Chagas' disease (Figure 3). CD86 expression was observed on the surface of some cells from samples with negative serology that were stimulated with LPS. Micrographs of culture of total autologous leukocytes, stimulation with LPS 30 minutes, in sample with positive serology for Chagas' disease, 400x CD80 expression in PMNs is shown.
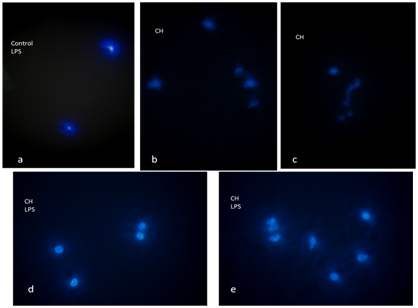
Figure 1 Representative micrographs of ETs, immunofluorescence, staining with DAPI (Sigma-Aldrich) for DNA in blue.
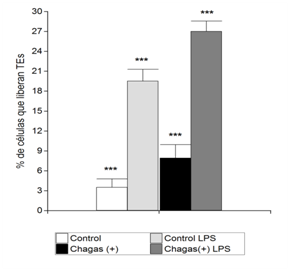
Figure 2 Percentage of cells that release ETs. The ETs were visualized by Microscopy of Immunofluorescence. The percentage of ETs released with LPS stimulation and no stimulation in samples with positive serology for Chagas' disease and control samples was calculated as the mean of six fields (400x) normalized with respect to the total number of cells. Data were presented as the mean ± standard deviation (n = 10) and represented at least three independent independent donor experiments. *** p <0.0001 for the Student t-test for paired samples and *** p <0.0001 for independent samples using the Infostat software.

Figure 3 Representative micrographs of co-stimulatory molecule expression in leukocytes in culture, immunofluorescence, staining with DAPI (Sigma-Aldrich) for DNA in blue; FITC (anti-CD-80, Santa Cruz Biotechnology) for CD80 in green; PE (anti-CD-86, Santa Cruz Biotechnology) for CD86 in red.
In the present study we observed the presence of ETs, both in samples of patients with positive serology for Chagas' disease without stimulation and stimulated with LPS. It is possible that these ETs occurred by the presence of the parasite, either by the presence of soluble antigens thereof, or by circulating antigen-antibody complexes. Although ETs constitute a defense mechanism, they have been implicated in tissue damage and related to autoimmune phenomena,14 which may be relevant to explain part of the pathophysiology of Chagas' disease. In a previous work, we observed the coexpression of costimulatory molecules in NETs in samples of healthy human blood.12 The presence of costimulatory molecules in NETs could influence the cellular environment resulting in stimulation or inhibition of other cells such as activated T cells or regulatory T cells. This would be important in the rupture of the immunotolerance described in the autoimmune phenomena present in the pathogenesis of Chagas' disease. T.cruzi infection has been shown to induce differential modulation of costimulatory molecules and cytokine expression in patients with Chagas' disease.13 In this work interestingly, when samples with Chagas-positive serology were stimulated with LPS neutrophils expressing CD80 were found and no CD86 positive cells were observed, in agree with the experimental in vitro study with T. cruzi and mononuclear cells.13 in that CD86 decreases its expression and increases expression of CD80 in patients with chronic chagasic cardiopathy.
In conclusion in the present study we observed the presence of ETs in leukocytes from patients with positive serology for Chagas' disease. What stimuli contribute to the formation of these ETs? It is possible that they were generated by the presence of the parasite, soluble antigens thereof, or by circulating antigen-antibody complexes. In addition, the presence of cytokines and inflammatory mediators in donor serum with Chagas-positive serology could induce the expression of costimulatory molecules in addition to LPS stimulation. The presence of these costimulatory molecules could be related to the possibility of rupture of self-tolerance in syntony with autoimmune phenomena described in Chagas' disease. In samples with Chagas-positive serology stimulated with LPS neutrophils expressing CD80 were found and it would induce an immunoregulation. Further studies are necessary to evaluate the functional significance of this finding and to elucidate the molecular mechanisms that underlie the clinical evolution of patients.
To donors of the Blood Bank of the Institute of Hematology and Hemotherapy of the National University of Cordoba, Argentina, for blood samples. Human blood samples with informed consent were used according to the procedures approved (R107/12) by the National Clinical Hospital Ethics Committee. Samples were donated by the Blood Bank of the Hematology and Hemotherapy Institute of the National University of Cordoba, Argentina.
The authors have no conflicts of interest.

©2017 Rodriguez, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.