MOJ
eISSN: 2373-4442


Research Article Volume 3 Issue 6
Department of Pediatrics, Division of Allergy and Immunology, Research and Training Hospital of Sakarya University, Turkey
Correspondence: Hany M Ibrahim, Immunology and Physiology Unit, Department of Zoology, Faculty of Science, Menoufia University, Shebin El-kom, Menoufia, Egypt,, Tel 02-0100-3689245, Fax 02-048-223568
Received: August 09, 2016 | Published: August 29, 2016
Citation: Ibrahim HM (2016) Evaluation of the Immunotoxic Effects of Sub-Chronic Doses of Lambda-Cyhalothrin in Murine Model. MOJ Immunol 3(6): 00108. DOI: 10.15406/moji.2016.03.00108
Lambda-cyhalothrin (LCT) is belonging to type II pyrethroid insecticides and utilized predominantly in Egypt to control a wide range of ectoparasites, including flies, mosquitoes, and cockroaches. Previous studies detected obvious genotoxicity and cytotoxicity of LCT. The current study was done to evaluate the immunological alterations on the levels of humoral and cellular immune responses after exposure to different doses (5, 1, 0.2mg/kg/day) of LCT in rat model. The current data displayed that LCT at dose 5mg/kg suppressed the total serum immunoglobulin concentration, hemagglutination titer, quantitative hemolysis of SRBCs (QHS), the phagocytic function of peritoneal macrophages and blood neutrophils. Moreover, 5mg/kg of LCT decreased the delayed-type hypersensitivity response, the proliferative response of the blood mononuclear cells, induced blood mononuclear cells death and reduced the percentage of CD4+ and CD8+ blood mononuclear cells without affecting CD4/CD8 ratio significantly. LCT at dose 1mg/kg inhibited the total serum immunoglobulin, QHS, the proliferative capacity of blood mononuclear cells, the phagocytic function of peritoneal macrophages and blood neutrophils. No significant differences were observed in all examined parameters in animals exposed to 0.2mg/kg of LCT. In conclusion, LCT exposure induced-immunotoxicity in a rat model at doses 5 and 1mg/kg, while 0.2mg/kg has no adverse effect on the examined immune parameters.
Keywords: lambda-cyhalothrin, NOAEL, BMCs proliferation, phagocytosis, immunoglobulin, cell death
LCT, lambda-cyhalothrin; LD, lethal dose; NOAEL, no-observed-adverse-effect level; Con A, concanavalin A; SRBCs, sheep red blood cells; WBCs, white blood cells; Ig, immunoglobulin; HA, hemagglutination; QHS, quantitative hemolysis of SRBCs; FCS, fetal calf serum; DTH, delayed-type of hypersensitivity response, BMCs, blood mononuclear cells; FITC, fluorescein iso-thiocyanate; ROS, reactive oxygen species; MDA: malondialdehyde
Synthetic pyrethroid insecticides, analogues of naturally occurring pyrethrins, can be structurally distinguished by presence of an alpha-cyano group substituent at the α-carbon of the phenoxy benzyl moiety (type II pyrethroid) or its absence (type I pyrethroid).1,2 Synthetic pyrethroids are selected over organochlorine, carbamate, and organophosphorus pesticides, because of their easy biodegradability, high efficacy, and low toxicity to mammals and birds.3 Cyhalothrin is belonging to type II pyrethroid insecticides and utilized on sheep, cattle and goats for the control of a wide range of ectoparasites, including flies, ticks and lice.4 Lambda-cyhalothrin (LCT) is one of the most commonly used insecticides in Egypt.5 LCT is used to control mosquitoes, flies, ants and cockroaches around houses. LCT has broad spectrum insecticidal activity as cyhalothrin, but it is more active than cyhalothrin.6 Many studies shed the light on vertebrate LCT induced-toxicity, including developmental toxicity,7 cytotoxicity, genotoxicity8 and endocrine disruption.9 Previous studies demonstrated LCT-toxicity in vitro, in rat bone marrow and human lymphocytes.8,10
The immune system contains highly specialized, memorized, complex cells, tissues and organs possess innate and adaptive responses to protect organisms from different pathogens. Epidemiological and experimental proofs demonstrated the intimate relation of the interactions between alterations in immune responses and stress factors.11 Several reports have been indicated the inducible stress-like symptoms of pyrethroid insecticides in experimental animals.12–14 Moreover, treatment of different animal models with type II pyrethroids such as deltamethrin, cypermethrin, fenvalerate and supercypermethrin forte, showed many immunosuppressive effects on humoral and cell-mediated immune response.15–18 Furthermore, some pyrethroid insecticides have been reported to cause spleen, thymus and lymph node damage as well as mutagenesis and carcinogenesis.19,20 Moreover, previous reports showed that cyhalothrin enhanced stress-like symptoms, and decreased phagocytosis and nitric oxide production by activated peritoneal macrophages.14,21
Adverse effects of insecticides on environment, animals and human health are an issue of public concern. LCT is wildly used in Egypt to spray on crops and around houses. Therefore, there is a dire need for a comprehensive research to realize the dose-dependent immunological alterations resulted by LCT. In the current study, doses of 5, 1 and 0.2 mg/kg of LCT were orally administrated to male rats. The oral LD50 value of LCT was estimated to be 79 mg/kg in male rats.22 The selected doses of LCT were at least 15 times smaller than those recorded for LD50 in male rats and therefore adequate for the search for an immunological no-observed-adverse-effect level (NOAEL). Moreover, recent study reported that 0.6 mg/kg of LCT elicited significant alterations in the level of malondialdehyde (MDA), nitric oxide, pro-inflammatory cytokines and up-regulated NF-κB/p65 in a rat model.23 Hence, 0.2 mg/kg of LCT, which considered three times lower than the dose that used in the previous study, could be especially convenient for the immunological NOAEL. Keeping this in view, the main goal of this study was to evaluate the immunotoxic effects of the selected sub-chronic doses of LCT in a rat model. The current study was extended to examine the suitable dose for the assessment of the NOAEL among the selected doses.
Chemicals
LCT (C23H19Cl-F3NO3) technical grade, purity 97%, was obtained from Help Pesticides and Chemical Company, Free zone, New Damietta, Egypt. The desired concentrations were prepared freshly when needed by diluting the LCT with corn oil. Concanavalin A (Con A) from Canavalia ensiformis [5 mg/ml stock solution in RPMI 1640 medium] was obtained from Sigma (Sigma, St Louis, MO, USA). All other chemicals and reagents were of the highest purity available.
o Wistar rats (weighting approximately 210-250 g) were obtained from the Egyptian Organization for Serology and Vaccination, Ministry of Health, Cairo, Egypt. For the preparation of complement which utilized in quantitative hemolysis of sheep red blood cells (SRBCs) assay, female guinea pigs (240g) were used. All animals were kept under planned laboratory conditions with a 12 hour dark/light cycle. Standard rodent food and clean water were supplied ad libitum. The animals were acclimatized to laboratory condition for at least ten days before the beginning of the experiments. The animals were used after consent of Institutional Animal Ethical Committee, Menoufia University.
Experimental design
The experimental animals were divided into four groups; twelve rats per group were used. Group I, control group, was orally received the vehicle, corn oil only, for 21 days. Group II, III, IV, experimental groups, were orally administrated convenient volumes of LCT solutions 5, 1 and 0.2 mg/kg per day, respectively, for the same period. At the end of the planned period, animals were anesthetized by i.p. injection with Avertin (Tribromoethanol, Sigma-Aldrich, 0.25 mg/g), dissected immediately, blood sample was collected from the hepatic portal vein of each animal. Each blood sample was divided into two tubes, one was mixed with EDTA or heparin and the other was permitted to clot. Samples were centrifuged at 3000 rpm for 15 min to detach blood serum. Serum was aliquoted and kept in deep-freezer at -30°C for further use.
Haematological parameters and granulocyte adhesion test
White blood cells (WBCs) total and relative differential counts were done manually using blood samples mixed with EDTA as described previously.24 After initial counts, one milliliter of blood sample was incubated with 80 mg of nylon fiber for 15 min at 37°C. The incubated blood samples were reanalyzed for total and differential counts. The obtained result of total leukocyte count and granulocyte percentage before and after incubation with nylon fiber gives granulocyte adhesion index of blood sample.25 Percentage of granulocyte adhesion was calculated as follows, granulocyte adhesion percentage = [(granulocyte absolute count before incubation with nylon fiber
ubtracted from granulocyte absolute count after incubation with nylon fiber) divided by granulocyte absolute count before incubation with nylon fiber] multiplied by 100.
Total Immunoglobulin (Ig) estimation
Total serum immunoglobulins were determined using zinc sulfate turbidity test.26,27 Briefly, twenty-five microliters of the examined serum were mixed with 1700µl of 0.7mM zinc sulfate, pH 5.8. The mixture was shaken and left to stand at room temperature for one hour. Serum mixed with PBS at the same ratio was utilized as the blank or control. Light absorption due to turbidity was measured photo-metrically at wavelength 545 nm to obtain the optical density of both test and control samples. Immunoglobulin levels of tested sera were obtained from calibration curve plotted on the basis of turbidity values corresponding to various dilutions of the standard serum sample.
Hemagglutination (HA) titer
SRBCs were collected in Alsever’s solution, then washed three times with large volumes of pyrogen-free sterile saline and stored for use. Eight days before the end of the experimental period, animals were immunized by i.p. injection of 1×108 SRBCs in saline. At the end of the experiment, collected serum samples were prepared and aliquots (twenty-five microliters) of two-fold diluted sera in saline were challenged with twenty-five microliters of 1% (v/v) SRBCs suspension in micro-titer plates. The plates were kept at 37°C for two hours and then observed for haemagglutination.28 The highest dilution giving haemagglutination was taken into consideration as antibody titer.
Quantitative hemolysis of SRBCs (QHS) assay
QHS assay was done using the method of Simpson and Gozzo with some modifications.29 Single cell suspension (1× 106 per ml) of the spleen was prepared in PBS. One milliliter of 0.2% SRBCs and one milliliter of 10% guinea pig serum were mixed with the previous cell suspension and incubated for one hour at 37°C. After centrifugation at 3000 rpm for 3 min, the optical density of the supernatants was determined at 413 nm using spectrophotometer (Helios alpha, Unicam, Leeds, UK).
Monolayer cultures of peritoneal macrophages
Peritoneal macrophages were collected from albino rats four days after i.p. injection of three milliliters of 4.05% brewer modified BBLTM thioglycolate medium (Becton Dickinson, Sparks, MD), by peritoneal washing with 5 ml of cold PBS. After harvesting, cells were centrifuged at 1000 rpm for 10 min and suspended in RPMI 1640 medium (Sigma) containing 10% fetal bovine serum. Then the macrophage suspension was added to twenty-four-well tissue culture micro-plates at density 1 × 106cells/well. The suspensions were kept at 37°C for three hours, washed thoroughly to remove non-adherent cells, and further incubated at 37°C.30
Assessment of phagocytic activity
In brief, neutrophils were isolated from heparinized blood samples.31 500 µl of the aliquot of cells (peritoneal macrophages or blood neutrophils) having density of 2×106 cells/ml was mixed with 500 µl RPMI-1640 containing 20% fetal calf serum (FCS) and 5×106 particle of the prepared zymosan solution. The mix was applied to a glass slide and incubated for two hours at 37°C in a humid chamber. The phagocytic index was determined by checking the Giemsa-stained phagocytic cells under the light microscope.32 The percentage of cells that engulf the zymosan was calculated after counting at least 100 cells in triplicates of each control and treated samples.
Delayed-type of hypersensitivity response (DTH)
Eight days before the end of the planned period of treatment, rats were primed i.p. with 0.1 ml of the SRBCs suspension containing 1×108 cells. Albino rats were challenged on day twenty with 1×108SRBCs in right-hind foot pad. The contra-lateral paw was given saline alone. The thickness of foot pad was measured at twenty-four hour after challenge using a micrometer (H. Kunkel, Offenbach, Germany). The difference in the thickness of right hind paw and left hind paw was used as a measurement of DTH response .33
The mononuclear cells isolation
Briefly, the mononuclear cells (the majority of cells are lymphocytes) were isolated by HISTOPAQUE®-1077 (Sigma) according to the manufacturer’s instructions.
Proliferation assay
Blood mononuclear cells (BMCs) proliferative responses to mitogen Con A were established by a micro-tissue
ulture system as described previously.27 BMCs were suspended in RPMI 1640 medium supplemented with 10% FCS, 100 IU/ml penicillin and 100 µg/ml streptomycin (Sigma). BMCs (5 × 105) from treated and control animals were cultured for twenty-four hour with Con A. Then ten microliter of Cell Counting Kit-8 (Sigma) reagent was added. After three hours of incubation at 37°C in 5% CO2, the optical density was measured at 450 nm (Seac, Radim Company, Italy). The proliferation percentage was calculated by dividing each value (control or tested) by the average mean of the control samples (vehicle) multiplied by 100.
Cell death detection
Cultured 1×106 BMCs were fixed and permeabilized with 70% ice-cold ethanol for at least one hour and then the cells washed twice in PBS. DNA was stained by incubating the cells at 37ᵒC for one hour in 40𝜇g/ml propidium iodide and one hundred 𝜇g/ml DNase-free RNase in PBS. Samples were analyzed by flow cytometry using a FACS
aliber flow cytometer (BD immune cytometry systems, San Jose, CA). The FL2 red fluorescence channel was assessed on a linear scale, and the percentage of cells undergoing cell death was determined as the percentage of hypo-diploid cells (sub G0/G1 peak) .34
Blood mononuclear cells sub-typing
BMCs sub-typing were done as previously described.27 Briefly, BMCs (1 × 106 cells/ml) in RPMI-1640 were prepared as mentioned above and after counting viable cells by Trypan-blue dye exclusion method, BMCs cellularity was specified. CD4+ and CD8+ cells were determined using a FACS caliber flow cytometer (BD immune cytometry systems, San Jose, CA) with Cell Quest Software for data acquisition and analysis using fluorescein iso-thiocyanate (FITC)-labelled anti-ouse CD4 and CD8 monoclonal antibodies (BD Biosciences, San Jose, CA).
Statistical analysis
For statistical analysis, SPSS (IBM SPSS statistics for Windows, Armonk, NY) computer program was utilized. The statistical analysis was performed by one-way ANOVA setting the probability level p<0.05. Post hoc analysis of group differences was carried out by LSD test. The treated groups were compared with control group and with each other.
Alterations in hematological parameters
No statistically significant changes were detected in total leukocyte count among all LCT treated groups compared to each other or to the control group (Table 1). LCT at dose 5mg/kg significantly reduced (p<0.05) the relative lymphocytes and monocytes count when compared to control group. On the other hand, LCT at dose 5mg/kg resulted in a significant elevation (p<0.05) in the relative granulocytes count as compared to control group. Doses 1mg/kg and 0.2mg/kg of LCT could not produce statistically significant changes as compared to vehicle control.
Parameter |
Control (Vehicle) |
LCT 5mg/kg |
LCT 1mg/kg |
LCT 0.2mg/kg |
WBCs mm3 |
7941.7±719.5 |
7658.3±712.9 |
7906.7±789.7 |
8033.3±725.7 |
Lymphocytes % |
63.67±1.43 |
59.50±1.06* |
61.17±0.79 |
64.67±1.67 |
Granulocytes % |
26.17±1.64 |
32.83±0.95* |
29.83±0.65 |
25.50±1.78 |
Monocytes % |
10.17±0.54 |
7.67±0.42* |
8.83±0.31 |
9.83±0.54 |
Table 1 Effects of LCT treatment on total and relative differential leukocytes counts in blood of albino rats for 21 days
A number of animals/group = 6, Data are expressed as mean ± standard error (SE).
* p<0.05 indicate significant difference compared to the control group (vehicle corn oil).
Changes in humoral immunity
LCT at dose 5mg/kg significantly reduced (p<0.01) the serum anti-SRBC titer compared to control animals (Table 2), while doses 1mg/kg or 0.2mg/kg of LCT could not produce any significant change as compared to the control group. Both doses 5mg/kg and 1mg/kg of LCT resulted in a significant decrease (p<0.05) in the total serum.
Munoglobulin concentration and QHS. No significant effect was demonstrated at dose 0.2mg/kg when compared to the control rats.
Parameter |
Control (Vehicle) |
LCT 5mg/kg |
LCT 1mg/kg |
LCT 0.2mg/kg |
Total Immunoglobulin Concentration (g/l) |
5.22±0.11 |
3.17±0.13* |
4.70±0.14* |
5.69±0.07 |
Hemagglutination Titer (Log2 titer) |
5.67±0.33 |
3.50±0.34* |
5.66±0.21 |
5.17±0.65 |
Quantitative Hemolysis of SRBCs (×106) |
0.186±0.008 |
0.132±0.001* |
0.177±0.003* |
0.180±0.003 |
Table 3 Alterations in humoral immune response of albino rats exposed to different doses of LCT for 21 days
A number of animals/group = 6, Data are expressed as mean ± standard error (SE).
* p<0.05 indicate significant difference compared to the control group (vehicle corn oil).
Alterations in phagocytic efficiency and granulocyte adhesion
Figure 1, illustrated the effect of LCT on the granulocytes adhesion and phagocytic capability of peritoneal macrophages and blood neutrophils, expressed as the phagocytic index. An obvious significant reduction (p<0.05) in the phagocytic percentage was observed in rats after the treatment with LCT at dose 5mg/kg and 1mg/kg when compared to control animals. No significant differences were detected in rats treated with LCT at dose 0.2mg/kg. Furthermore, treatment with all doses of LCT showed no significant changes in the level of granulocytes adhesion when compared to control animals.
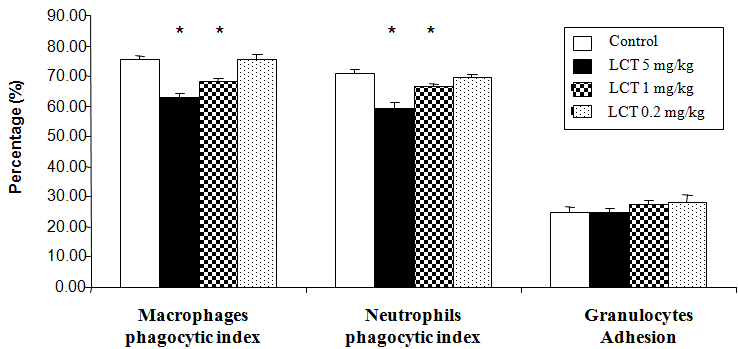
Figure 1 Changes in non-specific cellular immunity of albino rats exposed to LCT for 21 days. Data are expressed as: mean ± standard error (SE).
*p<0.05 indicate significant difference compared to the control group (vehicle corn oil), n=6.
Alterations in BMCs proliferation, death, and delayed-type hypersensitivity response
Delayed-type hypersensitivity response, BMCs death and the proliferative response of the BMCs (mainly lymphocytes) to mitogens such as Con A, an important phenomenon of cells maintenance and survival, was investigated in the current study (Figure 2). LCT at doses of 5mg/kg and 1mg/kg displayed a significant decrease (p<0.05) in the proliferative response of the BMCs as compared to the control group. However, LCT at dose of 0.2mg/kg did not show any significant difference when compared to the control animals. The results also indicated that LCT at dose 5mg/kg significantly suppressed (p<0.05) the DTH response and induced the BMCs death. LCT at the other doses (1mg/kg and 0.2mg/kg) did not display any significant change in the level of these parameters when compared to the control group.
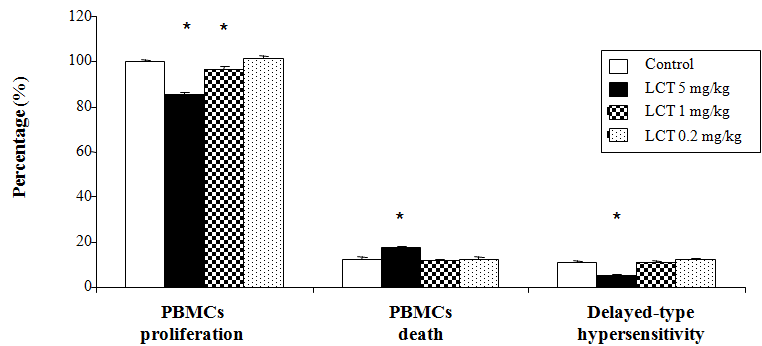
Figure 2 Changes in cell-mediated immunity of albino rats exposed to LCT for 21 days.
Data are expressed as: mean ± standard error (SE).
*p<0.05 indicate significant difference compared to the control group (vehicle corn oil), n=6.
Blood mononuclear cells sub-typing
LCT at dose of 5mg/kg per day significantly reduced (p<0.05) the percentage of CD4+ and CD8+ cells without influencing CD4/CD8 ratio (Table 3). However, LCT did not demonstrate any significant difference at doses 1mg/kg and 0.2mg/kg as compared to the control rats.
Treatment Groups |
CD4+ Cells (%) |
CD8+ Cells (%) |
CD4/CD8 Ratio |
Control |
64.59±2.04 |
21.78±0.95 |
2.98±0.54 |
LCT 5mg/kg |
43.53±1.37* |
14.70±0.95* |
3.00±0.14 |
LCT 1mg/kg |
63.65±1.43 |
21.69±0.98 |
2.95±0.08 |
LCT 0.2mg/kg |
66.26±1.15 |
22.24±0.97 |
2.99±0.08 |
Table 3 Alterations of blood CD4+ and CD8+ cells percentage of albino rats exposed to different doses of LCT for 21 days
A number of animals/group = 6, Data are expressed as mean ± standard error (SE).
* p<0.05 indicate significant difference compared to the control group (vehicle corn oil).
It is well established that there are intimate interactions among the nervous system, endocrine system and immune system which could be a potential target for both nervous and endocrine disruptors. LCT act on the central nervous
System and resulted in endocrine disruption.35,36 Therefore, like other xenobiotic LCT could be able to indirectly enhancing the immune alterations, by disrupting estrogen or affecting the signal from the nerve terminals to lymphocytes.36,37 Several lines of evidence indicated that pyrethroids, that belong to type II, were potent inducers of adrenaline and noradrenaline release and displayed significant estrogenic activity.36,38,39 The binding of specific adrenergic receptors on immune cells with noradrenaline modulated immune responses, mediated apoptosis and alters cytokine production.40–42 Moreover, estrogen might indirectly modulate the immune response by modifying the patterns of cytokine and induced apoptosis by several pathways.43 A recent study demonstrated that LCT-intoxicated rats elicited significant up-regulation of NF-κB/p65 and pro-inflammatory cytokine genes and increase their levels.23 It is generally accepted that pro-inflammatory cytokines, that could alter immune cell function, were the major effectors of apoptosis.44,45
In the current study, the high dose (5mg/kg) of LCT resulted in a significant decrease in the relative lymphocytes and monocytes count accompanied by an increase in the relative granulocytes count when compared to the control
nimals. These results were in accordance with the findings that reported by Basir et al.,46 in the female rabbits.46 The other doses (1mg/kg and 0.2mg/kg) did not display any significant changes in the level of these parameters. Moreover, the current data displayed obvious reduction in the cell-mediated immunity. The high dose 5mg/kg of LCT decreased DTH response, the percentages of CD4+ cells, CD8+ cells and the proliferative response of blood mononuclear cells, while enhanced BMCs death. The moderate dose 1mg/kg of LCT significantly reduced only the proliferative response of BMCs to mitogen when compared to the control animals. These results were in harmony with previous reports as follows. Obvious reduction in the DTH reaction to tuberculin and lymphopenia were evident in rabbits, which were treated with LCT.47 Celik et al.,8 reported that LCT is a potent inhibitor of mitosis as exposure to LCT caused inhibition of the cell proliferation of rat bone marrow.8DNA damage and reduction in the viability of human lymphocytes exposed to LCT was detected in a dose-dependent way.10 Thus, the obvious decrease in the mononuclear immune cells after exposure to LCT could be resulted from the LCT ability to reduce the BMCs proliferation and enhance the cellular death that may be happened through its neurological and endocrine responses.
In the present study significant inhibition in humoral immune response was detected in animals treated with high dose (5mg/kg) of LCT. The moderate dose 1mg/kg significantly reduced total immunoglobulin concentration and QHS. The detected reduction of humoral immune response assured the immunosuppression demonstrated after exposure to type II pyrethroids in the murine model.15,48,49 Bhoopendra and Nitesh (2014) demonstrated humoral immune response depression accompanied by a decrease in the peripheral B lymphocytes percentage and spleen atrophic changes in the white pulps which characterized by the decreased size of peri-arteriolar lymphoid sheath and lymphoid follicles in rats exposed to 10 mg/kg of LCT.20 Moreover, LCT exposure to rabbits elicited reduction in the serum hemolysin titer in dose-dependent manner.47
Nonspecific cellular immune response that examined in the current study showed significant inhibition in the phagocytic activity of blood neutrophils and peritoneal macrophages in LCT-treated rats (high dose 5mg/kg and moderate dose 1mg/kg) as compared to the control animals. On the other hand, no significant differences were recorded at the level of granulocytes adhesion in rats treated with different doses of LCT. In this respect several in ivo and in vitro studies have been done to evaluate the effect of cyhalothrin and LCT on phagocytic cells. Cyhalothrin decreased oxidative burst, nitric oxide production, the intensity, and percentage of phagocytosis in peritoneal macrophages.11,21,50 In a dose-dependent way, LCT-induced macrophage cell line RAW 264.7 cytotoxicity, decreased the cells viability and enhanced its apoptosis, through LCT estrogenic activity or its ability to increase reactive oxygen species (ROS) and DNA damage.36,51
Besides the ability of LCT to alter the immune response through its neurological and endocrine responses, different scenarios could also explain the immunological alteration that resulted from the exposure to LCT. One scenario based on the direct genotoxicity of the LCD. The immunotoxic effect of LCT could be attributed to its specific genotoxicity.8 In vitro study demonstrated the LCT genotoxic effects on human lymphocytes.10 The other scenario related to the LCT oxidative stress. The oxidative damage could be resulted in cytotoxicity and immune alterations. Several previous reports recorded the generation of ROS, impairment of antioxidant enzyme activities and increased MDA and nitric oxide levels after exposure to LCT.21,23,52–56 It is possible that one scenario or more have contributed to the immunotoxic effects induced by LCT.
Based on the experimental finding of the current study, it is suggested that LCT exposure induced immunotoxicity in albino rats. The presented data demonstrated that the LCT low dose (0.2mg/kg) has no immunotoxic effect in the rat model. Subsequently, 0.2mg/kg of LCT seems to be suitable dosage for the assessment of the NOAEL.
None.
The Author declares no conflict of interest related to this work and did not receive any fund for this study.
None.

©2016 Ibrahim. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.