MOJ
eISSN: 2373-4442


Research Article Volume 5 Issue 3
1Student Research Committee, School of Nutrition and Food Science, Tabriz University of Medical Sciences, Iran
2Department of Immunology, Faculty of Medicine, Tabriz University of Medical Sciences, Iran
3Nutrition Research Center, Department of Community Nutrition, School of Nutrition, Tabriz University of Medical Sciences, Iran
Correspondence: Meisam Barati, Nutrition Research Center, Department of Community Nutrition, School of Nutrition, Tabriz University of Medical Sciences, Attar Neishaboori St, Golgasht Ave, Tabriz, Iran, Tel 989-141-005-681, Fax 984-133-371-971
Received: January 01, 1971 | Published: March 23, 2017
Citation: Barati M, Yousefi M, Mohammdi H, Ebrahimi-Mameghani M (2017) The Immune Response Against Oryzatensin Increase VEGF and MMP-9 Experssion in HEP-G2 Cell Line. MOJ Immunol 5(3): 00159. DOI: 10.15406/moji.2017.05.00159
Background: Previous studies introduced ORZ for pharmacological purposes. As ORZ reduces NKs originated IFN-γ potentially. Therefore this study was designed to evaluate VEGF and MMP-9 expression in HEP-G2 cell line after culture with the soup of ORZ treated PBMCs.
Materials and methods: In current in-vitro study, PBMCs from seven healthy male subjects (aged 20 to 30 years) were isolated by ficoll density gradient. The soup of ORZ treated PBMCs were cultured with HEP-G2 cell line and the VEGF and MMP-9 expression in the cancerous cell line were measured by real time PCR.
Results: VEGF and MMP-9 expression increased after 48 hours, but only the increment of MMP-9 expression was statistically significant (P<0.05). The expression of these genes did not alter significantly after 24h and 72h incubation of the cancer cells with the soup of ORZ treated PBMCs soup (P>0.05).
Conclusion: By considering the increase of MMP-9 and VEGF expression in the cancerous cell line cultured with ORZ-stimulated PBMCs soup, it seems long term administration of ORZ, as a drug, may change the response of immune system toward carcinogenesis.
Keywords:oryzatensin, bioactive peptide, immune system, cell proliferation, oryza
ORZ, oryzatensin; MMP-9, matrix metalloproteinase 9; PBMCs, peripheral blood mononuclear cell; VEGF, vascular endothelial growth factor; NKs, natural killer cells, PCR, polymerase chain reaction
ORZ (amino acid sequence: Gly-Tyr-Pro-Met-Tyr-Pro-Leu-Pro-Arg) is a digestion resistant peptide elicited from brown rice (molecular weight= 1093.3). The brown rice bio-active peptide illustrate various functions such as Polymorphonuclear cells stimulation, activation of C3a (one of the complement system components) receptor (C3aR) and the ileum contraction.1,2 ORZ activates C3aR and imitates C3a function. The ORZ modification was firstly performed to produce a digestion resistant peptide with enhanced affinity to C3aR. Oral administration of modified ORZ, through the digestive system, induces a number of relatively important functions such as anti-amnesic, anorexigenic and anti-analgesic ones.3,4
Peripheral blood mono-nuclear cells (PBMCs) contain natural killer cells (NKs), T lymphocytes, B lymphocytes and monocytes. NKs bind to C3a and C5a by C3aR.5 Activation of C3aR on NKs reduces NKs originated interferon γ (IFN-γ).5,6 IFN-γ has been shown to have a pivotal role in Thelper1/Thelper2 (Th1/Th2) skewing. Therefore, IFN-γ reduction makes the condition in favor of Th2 activation.7,8 Th1 with IFN- γ together can induce apoptosis in cancer cells.9 Vascular Endothelial Growth Factor (VEGF) is a regulator of angiogenesis during different conditions such as embryogenesis, tumorigenesis, etc. Matrix Metalloproteinase 9 (MMP-9) belongs to MMPs that also have role in angiogenesis by destruction of extra-cellular matrix. Both VEGF and the MMPs have role in progression of cancer.10,11 As studies assessing the effects of immune response versus food component on cancer progression is rare, this study was designed to investigate the effects of immune response against ORZ, as a food component, on cancer progression In-vitro.
PBMCs Isolation, MTT assay and co-culture
Seven healthy male volunteers aged 20-30 years were recruited in the current study which approved by the Ethic Committee of Tabriz University of Medical Sciences and written informed consent was obtained from each subject. In this study, all tests on human participants were in accordance with the ethical standards of the institutional committee as well as the 1964 Helsinki declaration and its later amendments (Ethics code: Tbzmed.rec-1393-3250). Blood samples were collected in heparinized tubes and diluted with RPMI-1640 containing antibiotics (Gibco, UK). Then PBMCs were obtained by centrifuging the blood samples on Ficoll-Hypaque and washed by RPMI-1640 containing antibiotic and finally, the pellet was suspended in RPMI-1640 containing 10% FBS (Gibco, USA) and the viability was assessed by Trypan blue staining. ORZ with 98.8% purity (Biomatic, USA) was purchased from Biomatic Corporation. The 4×106 PBMCs were cultured in exposed with ORZ (the final ORZ concentration was 10-5 M). The PBMCs were cultured without ORZ, as control. The media of both groups were separated from PBMCs after 72 hours incubation. HEP-G2 cell line was purchased from Pasteur Institute of Iran. In order to culture of HEP-G2 cells with the soup of ORZ stimulated PBMCs, 5×105 HEP-G2 cells were seeded in 25mm flask and after 24h of incubation, the cancerous cells were washed with PBS. HEP-G2 cells were cultured in the Isolated PBMCs soup from both treatment and control groups in the ratio of 1:1 (5ml PBMCs soup+ 5ml fresh medium). After 24h, 48h and 72h incubation of cancerous cells with the PBMCs soup, the medium was removed then the cells were stored at -70°C until RNA extraction.
Total RNA was extracted from Hep-G2 cells at 24h, 48h and 72h after incubation using Thermo Scientific Gene JET RNA Purification Kit (K0731) according to the manufacturer's instruction. Extracted RNA was dissolved in nuclease-free water. RNA purification was assessed by NanoDrop Spectrophotometers. The integrity of RNA samples were assessed using 1% standard agarose gel. Reverse transcription was carried out using the RevertAidTM first strand cDNA synthesis kit (K1622; Fermentas, Germany). Two µg RNA was used for the first-strand cDNA synthesis in a total volume of 20 µL according to the manufacturer's guidelines. All PCRs were done using the Corbett Rotor-Gene™ 6000 HRM (Corbett Research, Australia) in a total volume of 20 µL containing Power SYBR Green master mix (2x) (TaKaRa Ex Taq HS, Japan), Primer, cDNA plus nuclease free water. The mRNA-specific primers were designed using Oligo 7 v.7.52 software (Molecular Biology Insights, Inc, USA). The sequences are listed in (Table 1). Each β-actin, MMP-9 and VEGF amplification were performed in triplicates for each sample. Delta CT values were calculated in relation to β-actin CT values by the 2-ΔΔCT method, in which ΔCT represents the difference between the CT value of target genes and the CT value of β-actin.
Genes |
Sequence (5' → 3') |
|
Β-actin |
Reverse |
GTAGTTTCGTGGATGCCACA |
forward |
TCCCTGGAGAAGAGCTACG |
|
VEGF |
Reverse |
GTGGGTGGGTGTGTCTACAGGAA |
forward |
CGCCACCACACCATCACCATC |
|
MMP-9 |
Reverse |
ATCCGGCAAACTGGCTCCTTC |
forward |
ATTTCTGCCAGGACCGCTTCTAC |
|
Table 1 The Primers Sequences
The data analysis was performed by SPSS software ver.21.0. The Kolmogrov-Sirnov test was used to assess the distribution of quantitative variables. One Sample T test was done for intra-groups statistical analysis. P-values less than 0.05 were considered statistically significant.
MMP-9 and VEGF expression were assessed in HEP-G2 cell line at 24h, 48h and 72h after incubation with soup of ORZ-stimulated-PBMCs. VEGF fold changes in first, second and third time points were 1.21±0.31, 1.45±0.38, and 1.25±0.23, respectively. In the all-time points the expression of VEGF increased but the increments were not statistically significant in comparison with the control groups (P>0.05). The expression of MMP-9, similar to VEGF, increased in the cancerous cells compared with control groups after the treatment. The over-expressions of MMP-9 in HEP-G2 at 24h and 72h after incubation were not statistically significant (P>0.05) while the treatment significantly increased MMP-9 expression after 48h incubation of HEP-G2 cell line with the soup of ORZ-treated PBMCs (P<0.05) (Figure 1–3).
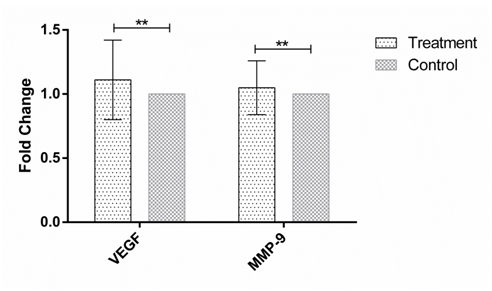
Figure 1 The expression of VEGF and MMP-9 in human Hep-G2 cell line after 24h incubation with the soup of ORZ exposed PBMCs. X-axis represents the genes and Y-axis shows fold change of the genes. Statistical analysis was done by One Sample T test. Each point represent the mean± SD. **P>0.05. VEGF: Vascular Endothelial Growth Factor. MMP-9: Matrix Metalloproteinase 9.
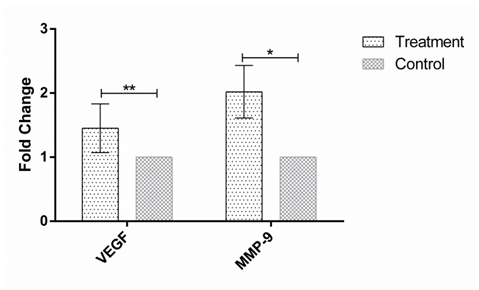
Figure 2 The expression of VEGF and MMP-9 in human Hep-G2 cell line after 48h incubation with the soup of ORZ exposed PBMCs. X-axis represents the genes and Y-axis shows fold change of the genes. Statistical analysis was done by One Sample T test. Each point represent the mean± SD. **P>0.05. *P<0.05. VEGF: Vascular Endothelial Growth Factor. MMP-9: Matrix Metalloproteinase 9.
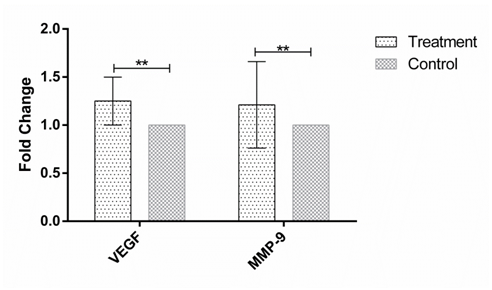
Figure 3 The expression of VEGF and MMP-9 in human Hep-G2 cell line after 72h incubation with the soup of ORZ exposed PBMCs. X-axis represents the genes and Y-axis shows fold change of the genes. Statistical analysis was done by One Sample T test. Each point represent the mean± SD. **P>0.05. VEGF: Vascular Endothelial Growth Factor. MMP-9: Matrix Metalloproteinase 9.
In the current study, we demonstrated that immune response against ORZ increase cancer progression In-vitro. ORZ like C3a can bind to C3aR and activate it.1,2 Receptor of C3a is a G protein coupled receptor and that have role in the complement system. Recently it has been accepted that PMNs express C3aR. NKs comprise about 15% of PBMCs.12,13 Min et al. in an In-vitro study revealed that NKs express C3aR to detect C3a and C5a. Also the authors demonstrated that C3a reduce INF-γ production by NKs.6,14–16 Cellular immune response is induced by Thl cells. Cancer patients have impaired cell mediated immunity. The impairment is associated with Thl to Th2 skewing. Th1 differentiation is dependent mainly to IFN-γ. IFN-γ promotes the CD4+ naïve T lymphocytes to differentiate toward the Th1 and inhibits the differentiation of Th2. Some mutations in the receptors of IFN-γ increase cancer risk.17–19
The over expression of MMP-9 and VEGF in cancer cells were seen in this study. Assessing the expression of mentioned genes in HEP-G2 cells after culture with the soup of ORZ stimulated PBMCs in this study proposed that the immune response versus ORZ may be Th2 based response in people aged 20-30 years. Th cells play a critical role in the immune system responses regulation. These cells based on the cytokines production subdivide to Th1 or Th2.20,21 Th1 based response increases IFN-γ production which in turn has direct effect on the proliferation of Th1. However it showed inverse effect on the proliferation of Th2.6,22,23 Th1 lymphocytes and IFN-γ, involve in anticancer mechanisms whereas Th2 response boost cancerous cells proliferation.9,24 VEGF plays critical role in the angiogenesis of tumor. Therefore, inhibition of VEGF action leads to the suppression of tumor growth. The function of VEGF in cancer progression is not limited to angiogenesis. MMP-9 strongly associated with tumors metastasis.10,25–27
The current study has some limitations. Subjects aged 20 to 30 years old individuals were recruited in the study and other age groups were not evaluated. Responses of immune system change during lifespan.28,29 In the other hand more experiments (i.e. ELISA and Flow cytometry) are necessary to confirm this conclusion.
By considering the increase of MMP-9 and VEGF expression in the cancerous cell line cultured with the soup of ORZ-stimulated PBMCs, it appeared that long term administration of ORZ, as a drug, may change the response of immune system and possibly could sensitize target populations to cancer.
The authors thank Tabriz University of Medical Sciences. This work was supported by Tabriz University of Medical Sciences.
The authors declare no conflicts of interest.
Not applicable.

©2017 Barati, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.