MOJ
eISSN: 2373-4442


Research Article Volume 5 Issue 2
1Department of Immunology, Pasteur Institute of Iran, Iran
2Department of Microbiology, Islamic Azad University, Iran
3Laboratory of Venom and Biotherapeutics Molecules, Biotechnology Department, Pasteur Institute of Iran, Iran
4Department of Immunology, Shahed University, Iran
5Department of Clinical Biochemistry, Shahid Beheshti University of Medical Sciences, Iran
6Department of Microbiology, Pharmaceutical Sciences Branch, Islamic Azad University, Iran
7Department of Immunology, School of Medicine, Mazandaran University of Medical Sciences, Iran
Correspondence: Mehdi Mahdavi, Departments of Immunology, Pasteur Institute of Iran, Tehran, Iran.69 Pasteur Ave. Kargar Ave. P.O.Box:1316943551 Tehran, Iran, Tel 982164112840, Fax 98 21 64112840
Received: January 01, 1971 | Published: February 17, 2017
Citation: Mahdavi M, Cheragh M, Bagheri KP, Adibzadeh MM, Tajik AH, et al. (2017) Adjuvant Effect of Royal Jelly on HIV-1 Multi-Epitope Vaccine Candidate: Induction of Th1 Cytokine Pattern. MOJ Immunol 5(2): 00152. DOI: 10.15406/moji.2017.05.00152
Studies show that, royal jelly as a naturally substance could improves immune responses. So, here, royal jelly as adjuvant was used in mixture with a recombinant multi–epitope HIV–1 vaccine model and cellular and humoral immune pattern was analyzed. Mice were immunized three times with recombinant HIV–1 vaccine that formulated in Royal jelly or mixture of Royal jelly/alum with two week interval. Then lymphocyte proliferation assessed with BrdU and IL–4, IFN–γ cytokines, total IgG antibodies and IgG1, IgG2a, IgG2b and IgM isotypes were assessed with ELISA. Results show Royal jelly as adjuvant increased lymphocyte proliferation and IFN–γ cytokine secretion versus control groups (P<0.05). Also, Royal jelly has increased total antibody and isotypes of antibody such as IgM, IgG1, IgG2a, and IgG2b (P<0.05). Overally, Royal jelly at dose of 10 µg could increase cellular and humoral immune responses alone and shows synergistic effect with alum adjuvant in the improvement of immunologic parameters. It is believed that bioactive molecules in Royal jelly could act as immunopotentiator as a part of mixture adjuvant and promote Th1 immune platform.
Keywords: adjuvant, th–1, immune response, recombinant hiv–1 vaccine, royal jelly
The best long–term hope for controlling AIDS is the development of a safe, effective, and affordable prophylactic vaccine. A major obstacle in developing an effective vaccine to stop AIDS–epidemic is genetic variation of HIV–1 virus. An ideal HIV–1 vaccine will need to stimulate both humoral and cellular immune responses against the virus.1 Up to now, there are no fully effective vaccines against HIV–1 and thus prevention of the infection requires the development of new vaccine technologies.2
The purpose of vaccination is to generate of a strong immune response to prevent or attenuate the virulence of pathogens.3 Different commercially available vaccine can be categorized into one of four types: live attenuated, killed inactivated, toxoid, and subunit.4 The new generation of vaccines such as subunit protein and DNA vaccines has a more defined composition with high purity and tolerability. These vaccines, which lack most of the properties of the original pathogen, are often less immunogenic than live–attenuated and whole–inactivated virus vaccines and thus require additional components such as adjuvant to help stimulate protective immunity based on antibodies and effector T cell functions.5–7 Current HIV–1 vaccine models comprise either recombinant proteins or synthetic HIV–1 multi–epitope peptide constructs. Therefore, they require an adjuvant to build up their immunogenicity.8,9
Among various strategies for the improvement of vaccine efficacy, use of an adjuvant has been a top choice with many successes.10 Adjuvants are defined as molecules, compounds, or macromolecular complexes that can boost the humoral or cellular immune response against antigens, but which should cause minimal toxicity.11,12 Furthermore, with the usage of adjuvants, less antigen and fewer injections are needed.5 The term adjuvant is derived from the Latin word adjuvare, meaning ‘to help’ or ‘to enhance’.3 Immunological adjuvants were originally described by Ramon in 1924 as “substances used in combination with a specific antigen that produce a more robust immune response than the antigen alone.13 In general, adjuvants can be classified as immune modulators or delivery vehicles, with some components sharing both properties.11 Mostly oil emulsions, lipopolysaccharides, polymers, saponins, liposomes, cytokines, ISCOMs (Immunostimulating complexes), alums, bacterial toxins etc., are common adjuvants under investigation.14 Successful vaccine development requires knowing which adjuvants to use and knowing how to formulate adjuvants and antigens to achieve stable, safe and immunogenic vaccines.15
The greatest challenge with using adjuvants in human vaccinations is that most of the adjuvant formulations are associated with many disadvantages such as high toxicity and adverse side effects.16 Until recently, however, only one type of adjuvant–aluminum salts had been widely used within licensed human vaccines in the US. These salts include aluminum hydroxide, aluminum phosphate, and alum.10 According to the comparative studies in humans and animals, aluminum is a weak adjuvant for induction of cellular immune responses.17 In addition to this, aluminum adjuvants have some limitations such as local reactions and ineffectiveness for some antigens.17 Therefore, novel adjuvants and formulations will be required.15,17 Royal jelly is a natural substance that can be selected as an adjuvant.
Royal jelly, a yellowish–white acidic highly viscous product secreted from the hypopharyngeal and mandibular glands in the head of worker honeybees (Apis mellifera), is involved in the sexual determination of the queen bee and used in the nutrition of larvae.18,19 Royal jelly contains a complex composition of proteins, amino acids, phenols, carbohydrates, minerals, vitamins and unsaturated fatty acids.20,21 Due to its complex composition, Royal jelly has a multitude of physiological effects such as anti–inflammatory, antitumor, anti–allergic, antibacterial, and antioxidant activities.22,23
In the present investigation, we employed Royal jelly as a natural adjuvant and assessed cellular and humoral immune responses versus multi–epitope HIV–1 vaccine candidate.
Mice
Six to eight–week old female inbred Balb/c mice (n=80) were purchased from Pasteur Institute of Iran (Karaj, Iran). The animals were kept in the animal house of Pasteur Institute with condition of temperature 20–22 °C and free access to the food and with the appropriate ventilation.
Royal jelly
Royal jelly was gifted by Dr. Pooria Ghasemi form laboratory of Venom and Biotherapeutics Molecules of Pasteur Institute of Iran and homogenized in the sterile PBS and used for vaccine formulation.
Vaccine preparation
Recombinant HIV–1tat/pol/gag/env protein was produced in E coli BL21 DE3 as reported previously.24 The vaccine was absorbed on Alum adjuvant and then mixed with 10, 50 or 100 µg of Royal jelly. In this case, each 200 µl of the vaccine contained 10 µg of vaccine candidate. Royal jelly used in three different concentrations of 10, 50 and100 µg. As well as to compare the adjuvant activity of Royal jelly, Freund's adjuvant and also alum were used as standard Th1 and Th2 adjuvant model to compare with Royal jelly adjuvant activity.
Experimental groups and vaccination of mice
Groups of mice were studied as follows which in this study, mice were divided into 12 distinct groups that each group consist of 6–7 mice and all them weight was almost the same
(Approximately 20 g) that In separate cages were placed. Vaccine dose was 10 µg and Injection in three days 0, 21 and 42 subcutaneously was done and three weeks after the final injection the immunologic parameters were assessed.
Lymphocyte proliferation assay
Three weeks after the third immunization, the spleens of mice were removed under the laminar hood class II and suspended in sterile cold PBS containing 2% FBS. RBCs were lysed with lysis buffer and cell suspension was adjusted to 2×106 cells per milliliters in RPMI 1640 (Gibco) supplemented with 10 % FBS, 4 mM L–glutamine, 1 mM sodium pyrovate, 100 µg/ml streptomycin and 100 IU/ml penicillin. Then 100 µl of cell suspension was dispensed into 96–well flat–bottom culture plates (SPL) and stimulated with 10 µg /ml of recombinant HIV–1tat/pol/gag/env protein. Phytohemagglutinin–A (5 μg/ml, Gibco) as positive control and un–stimulated wells as negative control were used. All experiments were done in triplicate. After 72 h of cell culture, 20 µl of BrdU (Roche, Germany) was added to each well and the plates were further incubated at 37°C for 24 h. After incubation, the plates were centrifuged at 300g for 15 min, the supernatant was carefully aspirated, the plates were dried and 200 ml of Fixation/denaturation buffer was added to each well for 30 min. The plates were aspirated and 100 ml of anti–Brdu HRP conjugate as a secondary antibody was added and incubated for 2 h. Afterward, the plates were washed 5 times with PBS, TMB as substrate was finally added to wells and incubated for 5 min in the dark at room temperature, and reaction was stopped by adding 100 ml of 2N H2SO4. The OD450 nm of each well was determined. The stimulation index (SI) was calculated according to the following formula: SI = OD450 of the stimulated wells /OD450 of the un–stimulated wells.
ELISA of cytokines
Three weeks after the final shooting, a total number of 4 × 106 spleen cells were placed on each well of the 24–well plate using complete RPMI 1640, stimulated in vitro with 10 μg/ml of the recombinant
HIV–1tat/pol/gag/env protein and incubated at 37 °C in 5% CO2 and in the other wells un–stimulated samples were prepared. Three days post in vitro immunization, supernatants were removed and the concentration of IFN–γ and IL–4 was estimated by ELISA Kit (Quantikine, R&D Systems, USA) according to the manufacturer’s instruction. The concentration of each sample (pg/ml) was calculated according to the standard curve and the absolute cytokine production of each mouse was calculated with subtract of the stimulated well with un–stimulated one.
ELISA of total antibodies and isotypes
Antibodies versus vaccine candidate were assessed by an optimized indirect ELISA method. Briefly, 100 µl of 10 µg/ml of the recombinant HIV–1tat/pol/gag/env protein in PBS was added into 96–well ELISA Maxisorp plates (Nunc, Naperville, IL) and incubated overnight at 4°C. The wells washed with PBS containing 0.05% Tween 20 (washing buffer) and blocked 1 h at 37°C with 5% skimmed milk in PBS (blocking buffer). Plates were washed with washing buffer and 100 µl of serial dilutions of 1/400 to 1/51200 of sera were added to each wells and incubated at 37 °C for 2h. The wells were washed five times with washing buffer and incubated for 2 h with 100 µl of 1/8000 dilution of anti–mouse conjugated to HRP (sigma, USA). The wells were washed five times and incubated 30 min with 100 µl of TMB substrate in the dark. The reaction was stopped with adding of 100 µl of 2N H2SO4 and color density was measured at OD450 nm with ELISA plate reader. Detection of specific IgG1, IgG2a, IgG2b and IgM subclasses was carried out by using goat anti–mouse IgG1, IgG2a, IgG2b and IgM secondary antibodies (Sigma, USA) according to the manufacture’s instruction.
Statistical analysis
All experiments were performed in triplicate, and the data was expressed as means ± S.D of each experiment. Then after the mean of each triplicate were utilized in statistical analysis. Student’s t–test was applied for comparison of the means of experimental groups and HSD test was used. Values of P < 0.05 and 95% were considered statistically significant.
Results of lymphocyte proliferation
Results of lymphocyte proliferation show that immunization with vaccine candidate with or without Royal jelly (10, 50 and100 µg) as adjuvant significantly increased lymphocyte proliferation versus control groups (Royal jelly and PBS) (P<0.05).
Immunization with candidate vaccine formulated with doses of 10, 50 and 100 µg of Royal jelly does not show any positive effect of lymphocyte proliferation (P>0.05) versus alum adjuvanted group. Lymphocyte proliferation at groups received vaccine formulated in mixture of Royal jelly at concentration of 10,50 and100 µg with alum shows highest lymphocyte proliferation at 10 µg of Royal jelly admixed with alum that shows significant differences versus alum formulated group and vaccine formulated with 10 µg Royal jelly group (P=0.006). However, at concentration of 50 and 100 µg of Royal jelly admixed with alum the proliferation was higher than alum adjuvanted group but statistically does not show significant differences versus alum adjuvanted group (P=0.076, P=0.347 respectively).
Overally, lymphocyte proliferation activity at freund’s adjuvanted group shows the highest response that shows significant differences versus all experimental groups (P<0.005) and next mixture of 10µg Royal jelly/alum as adjuvant was the best one at mixture groups (Figure 1).

Figure 1 Lymphocyte proliferation of experimental groups according to stimulation index. Immunization with HIV-1tat/pol/gag/env vaccine with Royal jelly (10, 50 and 100 µg) significantly increased lymphocyte proliferation versus HIV-1tat/pol/gag/env vaccine group (P<0.05).
Results of IFN–γ cytokine assay
Result of IFN–γ cytokine in the experimental groups shows that injection of vaccine candidate adjuvanted in alum , freund’s Royal jelly and mixture of Royal jelly/alum significantly increased IFN–γ cytokine secretion versus control groups ( P<0.015).
Immunization with vaccine formulated with 10, 50 and 100 µg of Royal jelly increased IFN–γ secretion versus alum and freund’s formulation vaccine in which at concentration of 10µg of Royal jelly shows significant differences versus alum and freund’s formulation of vaccine (P<0.009). But at dose of 50µg shows significant differences versus alum adjuvanted group (P=0.013) but not freund’s adjuvanted group (P=0.332) and at dose of 100 of Royal jelly in vaccine formulation significant differences versus alum adjuvanted group (P=0.022) was observed while no significant differences was observed versus freund’s adjuvanted group (P=0.443). Immunization with vaccine formulated with 50 and 100µg of Royal jelly admixed with alum shows increase of IFN–γ secretion versus alum and freund’s formulation vaccine in which at concentration of 50 µg of Royal jelly shows significant differences versus alum (P=0.021), but not versus freund’s formulation of vaccine (P=0.428). At dose of 100 µg of Royal jelly, shows significant differences versus alum and freund’s adjuvanted group (P<0.007).
Immunization with mixture of Royal jelly/alum as adjuvant only at concentration of 100 µg of Royal jelly shows significant differences versus vaccine formulated with Royal jelly at concentration of 100 µg (P=0.036) (Figure 2a).
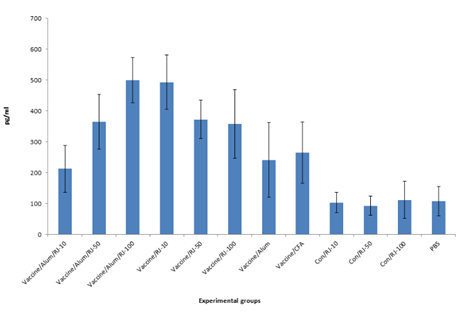
Figure 2a Assessment of IFN-γ cytokine in the experimental groups. Immunization with HIV-1tat/pol/gag/env vaccine with Royal jelly, alum, freund and mixture of Royal jelly/alum significantly increased IFN-γ cytokine secretion versus control groups. Immunization with vaccine formulated with 10 µg of Royal jelly significantly increased IFN-γ secretion versus alum and freund’s formulations (P<0.009).
Results of IL–4 cytokine assay
Result of IL–4 cytokine in the experimental groups shows that injection with all formulations of vaccine candidate (alum, freund’s, Royal jelly and Royal jelly/alum mixture) significantly increased IL–4 cytokine secretion versus control groups (P<0.017). Highest level of Il–4 cytokine secretion was observed in the groups that vaccine formulated with mixture of 10µg of Royal jelly’s and alum adjuvant but does not show significant differences versus other vaccine immunized group (P>0.05) (Figure 2b).
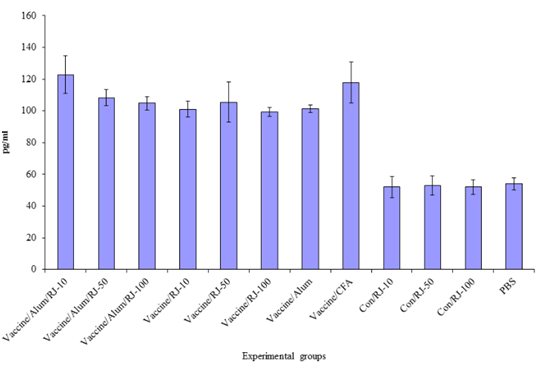
Figure 2b Result of IL-4 cytokine analysis after immunization course. Highest level of Il-4 cytokine secretion was observed in the groups that vaccine formulated with 10 µg of Royal jelly and alum adjuvant in mixture form.
Results of total antibody
Results of total antibodies show that immunization with all formulations of candidate vaccine induced specific antibody with significant differences versus control groups (P<0.014).
At dilution of 1/400 there is significant differences between Immunization of vaccine adjuvanted with Royal jelly/Alum mixture in comparison to Royal jelly’s and Alum’s adjuvanted vaccine at dose of 10µg (P<0.005). In generally, result of total antibody indicates that vaccine adjuvanted with alum/Royal jelly mixture at dose of 10µg of Royal jelly shows higher antibody response while, vaccine adjuvanted with Royal jelly at dose of 100µg of Royal jelly shows higher antibody response. Also humoral response of vaccine formulated with alum/royal jelly mixture at doses of 10µg of royal jelly was comparable to Freund adjuvanted vaccine at all dilutions and even was higher than Freund adjuvanted vaccine at dilutions of 400, 800, 1600 and 3200 (Figure 3).

Figure 3 Assessment of total antibody in the experimental groups. Result of total antibody demonstrates that vaccine adjuvanted with alum/Royal jelly mixture at dose of 10 µg of Royal jelly shows highest antibody response. At dilution of 1/400 there is significant differences between administration of vaccine adjuvanted with Royal jelly /alum mixture (10 µg of Royal jelly in mixture) as compared with Royal jelly and alum adjuvanted vaccines (P<0.005).
Results of specific IgG1, IgG2a, IgG2b and IgM isotypes
There are significant differences between HIV–1 formulated vaccines in experimental groups and control groups in the induction of specific IgG1 response (P≤0.014). There are significant increase of IgG1 response between immunization of vaccine adjuvanted with Royal jelly/alum mixture at doses of 10, 50 and 100 µg of
royal jelly as compared with Royal jelly’s adjuvanted vaccine at doses of 50 and 100µg and also alum and freund’s adjuvanted vaccines (P≤0.005) (Figure 4a).
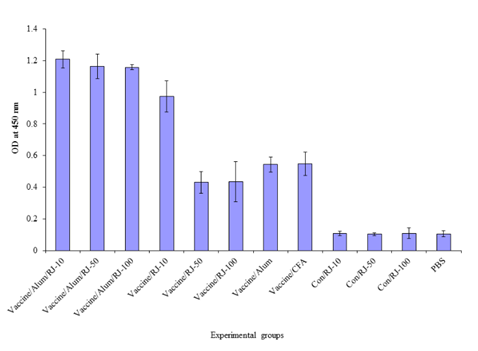
Figure 4a ThreeEvaluation of specific IgG1 isotype in the experimental groups. Results show that there are significant increase of IgG1 response between administration of vaccine adjuvanted with Royal jelly/alum mixture at doses of 10, 50 and 100 µg of Royal jelly as compared with Royal jelly adjuvanted vaccine at doses of 50 and 100 µg and also alum and freund’s adjuvanted vaccines (P≤0.005).
Results of specific IgG2a response shows that There are significant differences between administration of vaccine adjuvanted with Royal jelly/alum mixture at all three doses show significant increase as compared with Royal jelly’s at doses of 50 and 100µg (P<0.05). Also vaccine adjuvanted with Royal jelly/alum mixture at dose of 10 µg of royal jelly shows significant differences versus alum and freund’s adjuvanted vaccine (P<0.05) (Figure 4b).

Figure 4b Evaluation of specific IgG2a isotype in the experimental groups. Results show that immunization of vaccine with adjuvanted Royal jelly/alum mixture at doses of 10, 50 and 100 show significant increase as comparison to Royal jelly’s at doses of 50 and 100 µg (P<0.05).
Results of specific IgG2b response show that immunization with vaccine adjuvanted with Royal jelly/alum mixture at doses of 10, 50 and 100 µg of royal jelly increased IgG2b response versus all other groups. The highest response was observed in the group that immunized with vaccine adjuvanted with Royal jelly/alum mixture at doses of 10 of royal jelly that shows significant increase versus vaccine formulated with royal jelly at all three doses and alum and freund’s adjuvanted vaccine (P<0.05) (Figure 4c).

Figure 4c Assessment of specific IgG2b isotype in the experimental groups. Results indicate that immunization of vaccine adjuvanted with Royal jelly/alum mixture at doses of 10 µg of Royal jelly that shows significant increase versus Royal jelly adjuvanted vaccine at all three doses as well as alum and freund’s adjuvanted vaccines (P<0.05). Also, vaccine adjuvanted with Royal jelly/alum mixture at doses of 10, 50 and 100 µg of Royal jelly increased IgG2b response versus all other groups.
Results of specific IgM response show that immunization with vaccine adjuvanted with Royal jelly/alum mixture at all doses of royal jelly induce highest IgM response versus all other groups. Immunization with vaccine adjuvanted with Royal jelly/alum mixture at doses of 10 of royal jelly shows significant increase of IgM versus vaccine formulated with royal jelly at all three doses (P<0.011) and alum and freund’s adjuvanted vaccine (P<0.005) (Figure 4d).

Figure 4d Assessment of specific IgM isotype in the experimental groups. Results show that administration of vaccine with adjuvanted Royal jelly/alum mixture at doses of 10 µg of Royal jelly shows significant increase of IgM versus vaccine formulated with Royal jelly at all three doses (P<0.011) and alum and freund’s adjuvanted vaccines (P<0.005).
In the present study, multi epitopes of HIV–1tat/env/pol/gag vaccine was used as a model to clarify the adjuvant activity of Royal jelly and also mixture of Royal jelly and alum adjuvant on the polarization to Th1 immune responses.
It is well known that many candidate vaccines are ineffective such as HIV–1 vaccines.25 and should be improved with new approaches such as formulation in new candidate adjuvants.26 So that, use of vaccine adjuvanted with some natural compounds may be useful for improvement of vaccine efficacy.27 Substances such as Royal jelly with various immunomodulatory effects are good candidate for such purpose.28,29
Herein, immunomodulatory effect of royal jelly on HIV–1 vaccine candidate in two conditions was studied. In the first royal jelly was admixed directly to the vaccine and in the second condition, alum/royal jelly mixture was used as adjuvant for the vaccine model.
Result of this study showed that utilization of Royal jelly as an adjuvant in vaccine formulation increased lymphocytes proliferation responses. In other hand, Royal jelly at dose of 10 µg has synergic effect with alum adjuvant in the induction of lymphocyte proliferation. Actually Royal jelly has reinforced alum effect on lymphocyte function.
It is well known that Alum adjuvant couldn’t induce cellular immune responses and it can induce mainly humoral immune responses.30 Martin et al.31 demonstrated that Apalbumin–1 as major protein of Royal jelly induces immune system and also increased TNFα cytokine, that has important role on the maturation of dendritic cells as key player on T cell activation.31 Gasic et al.28 have shown that water extraction of Royal jelly increases T lymphocyte responses.
Lymphocyte proliferation reflects the function of cellular immune responses.32 and result of lymphocyte proliferation confirms the potency of Royal jelly on the induction of cellular immune responses. Studies show that 10–Hydroxy–trans–2–decenoic acid, the principal lipid component of Royal jelly is an strong activator of innate immune system that thereby could affects on T lymphocyte function.33 Kimura et al.34 reported the role of apisin, glycoprotein in Royal jelly, which stimulates human monocytes which as innate immune cell differentiated to macrophages that activate T lymphocytes.
Results of cytokine assay show that Royal jelly as an adjuvant increased IFN–γ release in both mixture form to alum/antigen and mixture with antigen. In mixture to alum 100 µg and in form of mixture to antigen 10 µg of Royal jelly shows best effect in the IFN–γ cytokine release. This result shows the potency of Royal jelly in the shift of immune response toward TH1 pattern. It meaning that Royal jelly has synergistic effect with alum in mixture form and is a potent adjuvant for polarization toward Th1 response while alum alone is failed to this. Dzopalic et al.35 have shown that 3, 10–Dihydroxy–decanoic acid purified from Royal jelly, promote human monocyte–derived dendritic cells to produce Th1 cytokine pattern.35 Induction of Th1 response is critical for combatting viral infection such as HIV–1. In fact, IFN–γ activates TCD8+ and TCD4+ lymphocytes against viral infections to eliminate infected cells.36,37 Alum as adjuvant fails to induce production of IFN–γ cytokine, while our study provide evidence that alum/Royal jelly as mixture adjuvant could induce production of IFN–γ cytokine and Th1 polarization. However, no obvious changes in IL–4 cytokine level was observed.
Antibodies play vital role in the neutralization of viruses before entry to the cells. So in the next, humoral immune responses were analyzed. Results show that Royal jelly at dose of 10 µg mixture with alum significantly increased total antibodies versus alum adjuvanted vaccine. This finding shows the synergistic effect of Royal jelly with alum in the enhancement of total antibody response which is important for neutralization of viral infection.38 Positive effect of Royal jelly on humoral response was shown previously by study of Sver et al. that confirm our finding.39
Further study on humoral immune response show that formulation of candidate vaccine in the mixture of alum/Royal jelly enhanced specific IgG1,IgG2a,IgG2b and IgM isotypes as compared with alum’s and freund’s and also Royal jelly adjuvanted vaccine.
These findings demonstrate that Royal jelly could induce all isotypes of antibody meaning poly–isotypic humoral immune response. This finding may be relate to the better induction of T cell immune response that in which result in better help to B lymphocytes. This subject is very important in respect to antibody function. Because, each certain isotype of antibody in process of humoral immunity has specific function. Stimulate antibody response with different isotypes is relation to powerful biologic activation.40
Overally, present study shows that Royal jelly as an adjuvant increased immune response versus HIV–1 vaccine model and mixture of Royal jelly with alum synergistically improved cellular and humoral immune responses. However we used total Royal jelly as an adjuvant, but we believe that bioactive molecules in Royal jelly act as immunopotentiator and in the future, further characterization of Royal jelly may result in finding of these molecules that would be useful as immunopotentiator for mixture adjuvant.
This work was supported in part by a grant from Pasteur Institute of Iran. We also thanks Dr. Kayhan Azadmanesh and Dr. Christine Hartoonian for technical support.
None.

©2017 Mahdavi, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.