MOJ
eISSN: 2373-4442


Case Report Volume 4 Issue 5
Rosalind Franklin University of Medicine and Science, USA
Correspondence: Dhauna P Karam, Rosalind Franklin University of Medicine and Science, 3333, Green bay rd, N. Chicago, IL 60064, USA, Tel 3098256959
Received: January 01, 1971 | Published: December 19, 2016
Citation: Karam DP, Morawiecki PA, Mouli CS, Agrawal B, Purohit PM (2016) Immune Thrombocytopenia in Association with Pancreatic Adenocarcinoma. MOJ Immunol 4(5): 00140. DOI: 10.15406/moji.2016.04.00140
Context: Evaluation for Immune thrombocytopenic purpura leading to diagnosis of advanced pancreatic adenocarcinoma has not been previously reported in literature.
Case report: We present a case of a 72 year old male who presented to our center with epistaxis, hematuria and prolonged bleeding from leg wound. Complete blood count revealed a platelet count of 2 k/uL. A complete physical exam with an extensive work up, ruled out other causes of thrombocytopenia including drugs, infections, liver disease and coagulation disorders. Computed tomography abdomen and pelvis, done as a part of workup to assess for hepatosplenomegaly revealed a pancreatic and a liver mass. Biopsy of liver mass confirmed the diagnosis of pancreatic adenocarcinoma. A trial of intravenous steroids and immunoglobulins quadrupled the platelet count initially, but then platelet count continued to drop. Further treatment with rituximab improved platelet counts to over 100 k/uL. The absence of any apparent cause of thrombocytopenia, coupled with a response to steroids and immunoglobulins initially, and rituximab later, were confirmatory of immune thrombocytopenia.
Conclusion: Immune thrombocytopenic purpura can be the presenting feature of advanced pancreatic adenocarcinoma and our case highlights the importance of a thorough workup that led to diagnosis of pancreatic malignancy.
Keywords: immune thrombocytopenia, pancreatic malignancy, itp, immune thrombocytopenic purpura, coagulation disorders, pancreatic cancer
Introduction
During the year 2016, in United States, it is estimated that 53,070 new cases of pancreatic adenocarcinoma will be diagnosed, out of which, 41, 780 people are expected to die of pancreatic cancer.1 In contrast to declining trend of incidence for most cancers, incidence of pancreatic cancer tends to be rising from 2003 to 2012 because of increasing prevalence of obesity, aging population and other unknown factors.2,3 Liver and pancreatic malignancies are considered two of the most fatal cancers with increasing cancer related death rates. Pancreatic cancer remains the fourth most common cause of cancer related deaths in men and women.4 Early detection of pancreatic cancer plays an important role in determining management and prognosis of this deadly disease.5 Here we present an interesting case of a patient in whom workup for ITP lead to diagnosis of pancreatic adenocarcinoma.
Case report
We present a case of a 72 year old male with the past medical history of successfully treated colon and bladder cancer, who presented to ER with complaints of epistaxis, hematuria and prolonged bleeding from superficial leg wound. Patient also complained of 40 pound weight loss in 2 months associated with mid abdominal and back pain. His vitals were stable. Physical exam was significant for multiple purpuric spots throughout the trunk and extremities with ecchymosis at IV insertion sites. Abdomen exam revealed non tender hepatosplenomegaly with a liver span of 14cm. Initial CBC revealed thrombocytopenia with platelet count of 2 k/uL, anemia with Hemoglobin of 11 g/dL and normal white blood cell count. Further review of the case history ruled out alcohol use, anticoagulants or liver cirrhosis as causes of thrombocytopenia. Peripheral smear showed no schistocytes. LDH, haptoglobulin and reticulocyte count were normal ruling out hemolysis. DIC was ruled out with normal fibrinogen level, Prothrombin and normal activated thromboplastin time.
Patient had no signs of infection and was not taking any medications that will cause thrombocytopenia (Figure 1). Pseudothrombocytopenia was ruled out by rechecking platelet counts in blood samples with sodium citrate as anticoagulant. Based on clinical findings and absence of other causes of thrombocytopenia including drug use, infection, liver cirrhosis, DIC, thrombotic platelet consuming disorders, pseudothrombocytopenia, acute thrombocytopenia was thought to be secondary to Immune etiology (Figure 2).
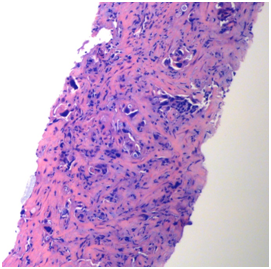
Figure 1 Core biopsy of liver mass demonstrating a poorly differentiated adenocarcinoma (10X, H and E).
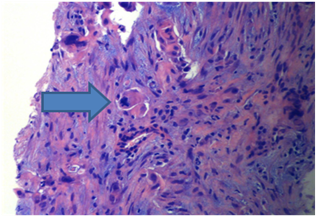
Figure 2 Tumor cells with nuclear pleomorphism and atypical mitotic figures (arrow) in a desmoplastic storm, (20X, H and E).
As a part of workup, a CT scan of abdomen/pelvis was ordered to evaluate hepatosplenomegaly, which revealed a large 9.5×11.6×2.4 cm mass lesion in the right lobe of liver with a 3×2×2.3 cm necrotic tumor in the distal body of pancreas. CT scan also revealed a moderate splenomegaly, of about 16.4 cm in length. MRI confirmed above findings and additionally revealed several adjacent satellite lesions along the lateral aspect of liver, the largest of which measured 2.8×1.8×2.6 cm. Carcinoembryonic antigen and alpha feto protein were within normal limits; CA-19-9 was elevated at 7114 U/mL. Biopsy of the liver lesion revealed pancreatic adenocarcinoma.
Patient was given a dose of intravenous steroids (solumedrol) and IVIG 1 mg/kg for management of ITP. Platelet count improved to 13 k/uL with above management and remained stable for 5 days. Patient was then transitioned to oral prednisone. Based on the clinical response, a diagnosis of immune thrombocytopenic purpura was confirmed and hence bone marrow exam or antibody analyses were not performed. But after initial improvement, platelet counts continued to drop while the patient was on oral steroids. Weekly rituximab was initiated, and counts increased to 100 k/uL. Platelet count remained stable thereafter. Before chemotherapy could be initiated, Patient chose hospice and died shortly thereafter (Figures 3&4).

Figure 4 Fine needle aspiration of liver demonstrating three dimensional groups of malignant cells with prominent nucleoli (20X, Pap stain).
Discussion
Immune thrombocytopenia (ITP) is an acquired form of thrombocytopenia due to autoantibody-mediated destruction of platelets.6 ITP can occur in children and adults; though it tends to be self-limiting in the former and chronic in the latter.6 The disorder is more common in young woman and older men above age 70.
ITP is a diagnosis of exclusion, characterized by isolated thrombocytopenia and the lack of a clinically-apparent condition responsible for the low platelet count. In the absence of disseminated intravascular coagulation, bone marrow infiltration or thrombotic microangiopathy causing thrombocytopenia, Immune mediated thrombocytopenia should be considered.7,8
In our patient, workup for new onset ITP revealed advanced pancreatic adenocarcinoma. We have long known the association of ITP with lymphoproliferative malignancies.9 and few solid tumors like non-small cell lung cancer, breast and ovarian cancers.10,11 To our knowledge, the occurrence of ITP as a possible paraneoplastic syndrome in a patient with pancreatic adenocarcinoma is rare with only two cases reported in literature.12,13 The etio-pathogenesis of such a correlation is not completely understood. Malignancies are known to activate auto immune cascade, which could lead to production of platelet autoantibody. Bir et al.12 reported that circulating immune complexes interact with platelets causing platelet aggregation and thrombocytopenia.
Steroids are the first line of treatment for ITP, followed by Intravenous immunoglobulins and rituximab. Splenectomy is reserved for patients not responding to conservative management.14-16
In our patient, the response to steroids and IVIG was transient. Sustained elevation in platelet counts were seen with rituximab infusion suggesting refractory ITP.
Our case highlights the importance of a prompt and complete workup of thrombocytopenia which could lead to diagnosis of occult malignancies.
A detailed history and physical exam, combined with appropriate diagnostic investigations, lead to the diagnosis of metastatic pancreatic malignancy in a patient presenting with thrombocytopenia. This highlights the importance of step wise approach in clinical practice.
The authors certify that, in the last 12 months, they do not have any financial relationships with any commercial organizations that might have influenced their contribution to the abstract. All authors completely agree with the abstract and its conclusions, and accept authorship on the abstract.
None.

©2016 Karam, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.