MOJ
eISSN: 2573-2919


Research Article Volume 3 Issue 3
1Eurovix S.p.A, Via Enrico Mattei, Italy
2Geoexplorer Impresa Sociale S.r.l., Italy
Correspondence: Enrico Guastaldi, Geoexplorer Impresa Sociale S.r.l., Via Bruxelles, 10, 52022-Cavriglia, Arezzo, Italy, Tel 39-055-9-1194-02; +39-349-6-1015 81, Fax 39-055-9119439
Received: May 11, 2018 | Published: June 12, 2018
Citation: Brogna F, Guastaldi E, Brignoli P. Use of bio–activators for the degradation of different soils contaminated by hydrocarbons. MOJ Eco Environ Sci. 2018;3(3):176-185. DOI: 10.15406/mojes.2018.03.00085
The overall aim of this paper is to understand whether there are differences in behavior between different types of soil, depending on their texture, regarding biodegradation in the event of environmental contamination by hydrocarbons. To test the possible inequalities of the different types of soil when subjected to contamination phenomena, a small–scale physical model was implemented by applying a biological degradation technique in the lab through the introduction of Micropan Alpha POBs, a particular bio–activator developed by the company Eurovix SpA, suitable for the biodegradation of soils contaminated by hydrocarbons (diesel), in order to speed up the degradation periods. Soil samples were taken from three sites located in Upper Valdarno (Tuscany, Italy). According to the results of this study, the key role in biodegradation processes is essentially by bacterial communities under specific conditions. The initial hydrocarbon concentrations decreased by at least 50% in the first month. Despite the in–depth and well–established studies on the ability of these bacterial communities to degrade hydrocarbon molecules, this research reveals how the geopedological study of an area is of added value. In fact, siltier soils analyzed during this research showed a higher predisposition to biodegradation, and both texture and water content and are leading factors for the soil biodegradation.
Keywords: hydrocarbons biodegradation, contaminated soils, microbial bio–activators, soil textures, geopedology
Oil bioremediation contaminated by petroleum hydrocarbons is a method established in recent years as one of the most environmentally sustainable reclamation technologies.1 In fact, it has been recognized as an inexpensive and highly efficient method of removing such compounds not only from soils but also from contaminated surfaces or underground waters.2–9 The key role of bacterial communities in biodegradation processes is essentially under specific environmental and climatic conditions. However, despite the extensive and well established studies on capacity of the hydrocarbon molecules that these bacterial communities are unable to degrade,10 this research finds that the geological–pedologic survey of contaminated soils is an added value when designing reclamation through bioremediation. In fact, knowledge of the genesis and evolution of soil from a contaminated site offers an effective contribution to the correct design of the drainage system in terms of timing, quantity of inoculable bio–degradation, etc., and provides assessments for improved soil management and preservation from degradation phenomena. The bioremediation of sites contaminated by organic compounds is based on the stimulation of the catabolic activity of microorganisms capable of using polluting organic contaminants as a source of carbon and energy. Organic compounds can therefore be completely degraded to carbon dioxide and water, i.e. mineralized or biotransformed into less toxic compounds.10 Specifically, the purpose of the research is to understand how different soil textures respond to natural and assisted biodegradation phenomena (or, through the inoculation of external bacterial communities). Similar studies have been conducted in recent years by other authors (for example: Haghollahi et al.11 & Kogbara et al.12). However, the present study introduces the geological–pedological analysis of the site and samples as an essential support to the design and the success of bioremediation. To test the possible differences in the behavior of the different types of soil when subjected to contamination phenomena, a biological degradation technique was applied to a reduced–scale laboratory model of three different types of soils (named A, B, C), using the Micro pan Alfa POBs compound, a particular bio–activator developed by Eurovix SpA, which is suitable for the treatment of contaminated soils (diesel) to speed up degradation times. The bioactivators used in this project consist essentially of a set of microorganisms, enzymes, supplements, nutrients, and growth factors that are developed for each type of application through the microbial selection process. This process leads to the formation of microbial consortia and thus to the execution of the microcosm/mesocosm test. These substances have already been tested and used in various soil contamination situations and, therefore, respond to the usage characteristics for research purposes.13–17 The specific aim of the work was to find out whether there could be differences in degradation times among the various contaminated soils from a biological point of view, or to evaluate the differences in degradation periods between samples with natural attenuation of contamination and the samples in which the bioactive substances were inoculated, from the textural point of view. Finally, this work is aimed to understand whether the different types of soil would respond differently to the environmental contamination phenomena depending on the granulometry.
The research consisted of sampling three types of soil and creating four sub–samples (aliquots) for each surface soil sample, one of which was maintained as an uncontaminated sample (control sample) and three of which was inoculated with the bio–activator. The work described is the result of several phases: the first phase involved the selection of sites to be sampled and their work on the field through the geological and geophysical study of the sampled terrains; the second was carried out in a laboratory where, after textural analysis of soil samples, a biodegradation test of hydrocarbons was set up on different soil samples and subsequent analysis of the results in terms of efficacy and degrading periods were reviewed.
Geological survey, soil sampling and textural analysis
The study area is characterized by a sub coastal temperate climate with a dry summer (such as Cs of the climatic classification of Köppen–Geiger), which affects the hilly areas of the Tosco–Umbrian–Marshal pre–Apennines and the lower slopes of the Southern Apennines (An average annual temperature of 10 °C to 14.4 °C and an average annual rainfall of about 850–1.050 mm) 18. Soil samples come from three distant sites, though they all pertain to Cavriglia in the Valdarno Superior area, not far from Florence, Siena and Arezzo (Tuscany, Italy). Although very similar, the choice for these three areas of study is that they have different characteristics in terms of texture and land use (agricultural, wooded and uncultivated areas) and can, therefore, be considered a representative selection of many areas from the Tuscan region. From a geological standpoint, soil samples taken for research purposes fall within the basin of Upper Valdarno (Figure 1A). On a regional scale, the Valdarno basin is inserted between the Polio–Quaternary mountain basins of the northern Apennines, embedded between the mountains of Chianti and Pratomagno upwards to SW to NE and delimited, lengthwise, by the cross of tectonic thresholds of Arezzo to the South and Incisa–Rignano to the North (indicated by some authors as Follonica–Rimini Line Piombino–Faenza)19. The rocks that make up the chain have settled in the paleogeographic domains that include the Ligurian–Piedmontese Ocean (Ligurian Domain), the African continental margin (Tuscan Domain and Umbro–Marchigiano Domain) and a transitional band interposed between them (Sub–Ligurian Domain). The tectonic units involved in the deformation of the chain and which emerge in the Chianti – Upper Valdarno – Pratomagno area are the Tuscan Units (Tuscan nappe, Falterona – Cervarola Units, referable to the Tuscan Domain) and the Ligurian Units (Morello Unit, Ligurian Domain) (Figure 1A), according to a widely described tectonic–structural model.20–25
Geological literature presents a general uniformity of interpretations regarding the three successive tectonic phases that generated the semi graben of Upper Valdarno, the first one in Pliocene and the other two in Pleistocene epoch, linked to regional–scale compressive tectonic events that are related to the discontinuities that define the different sedimentation cycles, 19,26–28 and who have left testimonials in the geometry of the same filling deposits. Each tectonic phase corresponds to the cyclic sedimentation of a fluvial and lacustrine environment, thicker than 500 m, which is in angular discordance with the sediments of the previous cycle. The study area as a whole is located at the base of the Upper Valdarno sediment basin, close to the pre–neogenic substrate along the southwestern bank of the basin itself (Figure 1A), where different soils attributed to different landscape units are located (Figure 1B). The study was conducted on the topsoil, as the surface is most affected by hydrocarbon contamination. The fundamental element for soil detection is the definition, distinction and characterization of the horizons, and consequently the relative sampling of the latter; in this way, it is possible to study both horizontal and vertical variations. The quantitative study of field observations and the qualitative analysis of laboratory tests are two aspects closely related to the definition of a soil.29 In this context, the study of pedology is very important because knowledge of the genesis and evolution of the soils, especially of the areas where the analyzed samples were taken, can contribute to bioremediation. The study areas in which soil samples have been chosen and retrieved are noticeably varied; in fact, there are several morphological environments and soil conditions that, when almost constant in a zone, they could be represented by a Landscape Unit (UdP). UdPs represent a landscape geological and pedological description ad level of Semi–detail (represented on scale 1: 25,000 – 1: 50,000), obtained from the Soil Map of the Tuscany Region on scale 1: 250,000,30 Detailed for the Arno river basin at level 2.31 Also, there are portions of territory within which the main factors of pedogenesis (lithology, physiography, soil use) are generally constant (Figure 1B). Three samples (A, B, C) were collected from different UdPs representative of a portion of Upper Valdarno, and divided into four aliquots of the same volume, three for the inoculation of microorganisms (aliquots A1, A2, A3; B1, B2, B3; C1, C2, C3), while the fourth to be maintained as control sample (ATQ, BTQ, CTQ) (Figure 1.B). Soil samples were taken from soil horizon A at a depth of 20 cm from the countryside. The soil sample A is classified according to the Soil Taxonomy as Lithic Ustorthents coarse–loamy, mixed, nonacid, mesic (2003) and as Eutri Epileptic Regosols (1998) according to the World Reference Base. Soil samples B and C are classified according to the Soil Taxonomy as Aquic Haplustepts, fine–loamy, mixed, mesic (2003) and as Stagnic Cambisols (1998) according to the World Reference Base.32
Laboratory test description
Soil samples were classified from the textural point of view through a series of laboratory protocols that allow one to obtain a granulometric classification. The most important element for the characterization of a soil is the texture that differentiates different types according to the percentages of Sand, Silt and Clay (Figure 2 and Figure 3). Furthermore, all samples were analyzed by a chemical laboratory according to the standard method UNI EN 14039:2005 for heavy hydrocarbons (C>12) quantifying. Moreover, several polycyclic aromatic hydrocarbon compounds were analyzed by means of UNI CEN/TS 16181:2013 standard method, however, their concentrations in soil samples were lower than detection limits. The first step of the biodegradation test was to contaminate the soil samples by heavy hydrocarbon (diesel) and it was chosen a sample of 1 kg for every type of soil in order to analyse and understand the initial concentrations of contaminants at time zero (August 2015). Subsequently, a physical model was built on a smaller scale, with the creation of four containers for each type of soil (one control sample and three contaminated samples) that were inoculated with bio–Micropan Alfa POBs. The composition of Micropan Alfa POBs was generically as follows: selected microorganisms (Trichoderma longibrachiatum Evx1 109 CFU g–1, Bacillus sp. 109CFU g–1), enzymatic components (i.e., laccase, Mn–peroxidase, cellulase, xylanase, protease, lipase, alpha– and beta–amylases) 2%, amino acids 10%, carbohydrates 12%, catalytic oligo elements 3%, Lithothamnium calcareum seaweed mat 5%, mineral salts 6 % and unspecified natural growth factors. The bio activator is formulated to be active on a wide range of pollutants and allows one to deal with problems relating to recovery of soil.33 It is a very effective compound on substances that are difficult to biodegrade and resistant to the action of indigenous microorganisms.34 The boxes where the bioactivator was inoculated were filled with 22 l of soil, and each box was inoculated with an amount of 1 g of bio activator per litre of soil for a total of about 22 g for each aliquot sample. During the test, the soil aliquot in which the bio activator was inoculated were turned and mixed every 3–5 days to allow oxygenation and favour the microbial activity of the bioactive substance. In addition, these aliquots have also been moistened to maintain humidity within a range between 15% to 50% water content, while the control sample was not turned.
The results obtained for each test sample are analyzed both from the geotechnical–tessutive point of view by the Miller Triangle32 and the point of view of the contaminant degradation. Sample A is characterized by a type of soil that can be used mainly mixed deciduous trees, secondarily by coniferous woods and, marginally, by arable soil and olive grove. The results obtained from the granulometric analysis show that soil sample A is poorly graded and it can be classified as “Loam – Sandy Loam”, sample B (more uniform grading curve) as “Silt Loam”, while sampling C (more graded grading curve) as “Silty Clay” (Figure 2) (Figure 3). The samples analyzed in the laboratory correspond to the pedological descriptions of UdP 61_3 (sample A) and 101_1 (samples B and C) (Figure 1.B). Specifically, relating to the pedological survey, sample A is characterized by a characteristic sequence of type ACR, sample B showed a characteristic sequence of type Ap–Bg–Cg–C, while sampling C by a characteristic sequence of type Ap–Bg–Cg–C. Those sequences also find correspondence in certain types of soil present in the Tuscan Region Soil map (Tuscany Region, 1999).
The three groups of analyzed samples exhibit different moisture values, especially the sandy sample A compared to the more silty samples B and C (Figures 6–8). Sample B shows an average moisture value around 30%, sample C around 40%, and sample A around 20%. This difference, probably linked to the type of granulometry that distinguishes the different samples, could affect the different degradation of the contaminant in the different samples. Various studies show the relationship between soil textures and hydrocarbon biodegradation phenomena. Ayotamuno et al.,35 show the effectiveness of fertilizer, water and tillage for bioremediation of silty clay soils. However, other authors11,12 show how sandy samples had better reacted to degradation than silty – clay soils. Indeed, various authors show that predominantly or completely claylike soils have low permeability and induce a delay in oxygen transport and nutrients compared to sandy soils.36 In addition, clays in particular conditions could also catalyze the formation of humic acids and slow down the degradation of organic compounds.37 Other authors, such as Schjønning et al.,38 argue that low levels of soil moisture content diminish microbiological activity, whereas an excess of water could create resistance to oxygen transfer and thus produce an undesirable leach. Nonetheless, the optimal soil water content, which is a function of soil and texture type, is not uniquely defined in the literature, but is certainly important for the effectiveness of bioremediation. In fact, both the granulometry, the texture and the water content of the samples used by the aforementioned authors11,12 are different from the parameters measured in our work, which are less classed and with a much lower content of water than theirs. In addition, the size of the experiment of Kogbara et al.,12 is inferior to that used by us, which however provides the arability of microhabitats for survival and activity of bacteria. From this we see the diversity of results obtained with respect to Haghollahi et al.,11 and the greater adherence of our experiment to the study area climate. Finally, the magnitude of soil degradation, even with the control sample, seems to be the same in the case of soils with non–negligible clay percentages. As far as the contaminant degradation test is assisted (through bioactivator inoculation to accelerate degrading periods) and natural (Natural Attenuation), the results clearly show how in the sample series A1, A2 and A3 , the initial concentration (August 2015, t0) decreased by almost 50% compared with the reduction measured in sample ATQ (Figure 7 and in Table 1), While for series B1, B2, B3 and C1, C2, C3 concentration was decreased (September 2015, T1) more than 50% respectively from sample BTQ (Table 1 and in Figure 7) is to sample CTQ (Table 1 and Figure 8). Furthermore, for C sample, a further chemical analysis was made about six months apart (February 2016, T2), whose results have shown that sample concentrations almost reached the regulatory limits set by Italian law, which for industrial and commercial sites it is 750 mg/kg ss (M.A.T.T., 2006), and the control sample concentrations reduced further than the initial values (Table 1) (Figure 8).
Furthermore, although B and C samples belong to the same UdP 101_1 (Figure 1 B), they have different granulometric curves. This difference can be explained by the fact that sample B was taken from a mature soil for agricultural use only, while sample C was taken from a younger soil formed after the landing of a portion of land carried out during the cultivation phase from the Lignite mine that came from that area. The different textures of the two soil samples could have led to the assumption that different timings and substantial variations on the degradation of the contaminant could occur, but this did not happen because the final test concentrations show similar values. Moreover, the analytical chemical method utilized for determining the hydrocarbon concentration could have increased the performances of the hydrocarbon adsorption capacity of silty soil compared to the sandy soil. For encompass this issue, the analyzes were repeated in different laboratories. Therefore, biodegradation, in addition to the presence, density and quality of the bacterial community capable of degrading hydrocarbons, would depend on the structure of the soil, i.e. its texture, its moisture content, its geomorphologic and microclimatic characteristics, which, as such, allowed to map that particular UdP.31 In addition, between the A (sandy) and the finest granulometry samples (B and C), there is a difference in terms of degradation, although all have the same trend of lowered hydrocarbon concentrations. Indeed, with regard to the siltier samples (B and C), at the same time the T1 and the TQ aliquots and those 1, 2 and 3 in which the microorganisms were inoculated showed lower concentrations than those found in sample A (sandy). This is in line with what has been stated in the previous point, i.e. that soils from different UdPs show different phenomena of biodegradation, noting in particular that the silty soils sampled are here more predisposed to biodegradation than those with greater granulometry.39
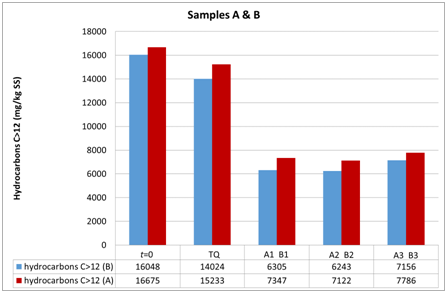
Figure 7 Evolution of hydrocarbon concentrations in soil samples A and B at the first (t0) and the second sampling (t1).
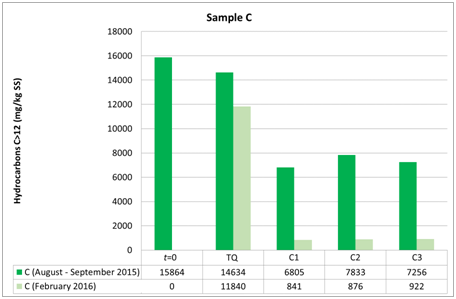
Figure 8 Evolution of hydrocarbon concentrations in soil samples C to the first (t0), the second (t1) and the third sampling (t2).
Sample ID |
Date of sampling |
Concentration of hydrocarbons C>12 (mg/kg SS) |
||||
|---|---|---|---|---|---|---|
t = 0 |
Aliquot |
|||||
TQ |
1 |
2 |
3 |
|||
A |
24/09/2015 |
16675 |
15233 |
7347 |
7122 |
7786 |
B |
24/09/2015 |
16048 |
14024 |
6305 |
6243 |
7156 |
C |
24/09/2015 |
15864 |
14634 |
6805 |
7833 |
7256 |
24/02/2016 |
15864 |
11840 |
841 |
876 |
922 |
|
Table 1 Values of hydrocarbon concentrations analyzed through UNI EN 14039:2005 methods in the soil samples
The results of the research have shown a difference in terms of degradation between the sandy and the finest samples, although all reveal the same trend of lowering hydrocarbon concentrations. Soils from different UdPs show different phenomena of biodegradation, noting in particular how the siltier soils analyzed during this research are more predisposed to biodegradation. Also, the results obtained from these samples can be considered representative only for the portion of the UdP relative in the study area, but not other portions located elsewhere. Thus, this study may have further developments that could lead to a regional estimate of the probability of biodegradation of soils similar to those sampled, increasing the number of samples to be tested on the basis of an optimal sampling pattern, extending then the results obtained to all areas covered by soils of the same kind as those analyzed. Moreover, other kinds of procedures for determining the hydrocarbon content in soil cannot could be utilized for quantifying the role played by the fraction of hydrocarbon adsorbed, which could have been mask by the analytical procedure utilized in this work. According to the results of this study, the key role in biodegradation processes is essentially by bacterial communities under specific conditions. However, despite the in–depth and well–established studies on the ability of these bacterial communities to degrade hydrocarbon molecules, this research reveals how the geopedological study of an area is of added value for selecting samples with similar characteristics that could lead to similar biodegradation results. Indeed, the knowledge of the genesis and the evolution of the soils of a site and its spatial distribution can make an effective contribution to the proper design of biodegradation (degradation periods, the quantity of bio activators inoculated, etc.), providing assessments for better soil management and preserving them from phenomena degradation in a particular site.
Sample A – sampling: September 24, 2015 |
|||||||
|---|---|---|---|---|---|---|---|
Parameter |
Method |
U.M |
t=0 |
TQ |
A |
B |
C |
hydrocarbons C>12 (A) |
UNI EN 14039:2005 |
mg/kg SS |
16675 |
15233 |
7347 |
7122 |
7786 |
Benzo (a) antracene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (a) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (b) fluorantene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (k) fluorantene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (g,h,i) perilene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Crisene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,e) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,l) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,h) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,h) antracene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Indeno(1,2,3–cd) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Sample B – sampling: September 24, 2015 |
|||||||
Parameter |
Method |
U.M |
t=0 |
TQ |
A |
B |
C |
hydrocarbons C>12 (B) |
UNI EN 14039:2005 |
mg/kg SS |
16048 |
14024 |
6305 |
6243 |
7156 |
Benzo (a) antracene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (a) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (b) fluorantene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (k) fluorantene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (g,h,i) perilene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Crisene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,e) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,l) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,h) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,h) antracene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Indeno(1,2,3–cd) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Sample C – sampling: September 24, 2015 and February 2016 |
|||||||
Parameter |
Method |
U.M |
t=0 |
TQ |
A |
B |
C |
hydrocarbons C>12 (C) |
UNI EN 14039:2005 |
mg/kg SS |
15864 |
14634 |
6805 |
7833 |
7256 |
hydrocarbons C>12 (C) |
UNI EN 14039:2005 |
mg/kg SS |
15864 |
11840 |
841 |
876 |
922 |
Benzo (a) antracene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (a) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (b) fluorantene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (k) fluorantene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Benzo (g,h,i)perilene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Crisene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,e) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,l) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,h) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Dibenzo (a,h) antracene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Indeno(1,2,3–cd) pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Pirene |
UNI CEN/TS 16181:2013 |
µg/kg SS |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
<0,02 |
Supplementary Table 1 Chemical results for all analites analyzed, method of analysis, measuring units and date of sampling
Grain size (mm) |
Sample |
||
|---|---|---|---|
A % passing |
B % passing |
C% passing |
|
100 |
100 |
100 |
100 |
75 |
100 |
100 |
100 |
50 |
100 |
100 |
100 |
37.5 |
100 |
100 |
100 |
25 |
98.62899158 |
100 |
100 |
19 |
96.81418266 |
100 |
100 |
9.5 |
96.50037407 |
100 |
100 |
4.75 |
96.29570074 |
99.83398632 |
99.82158 |
2 |
93.26281412 |
99.50195896 |
98.48977 |
0.85 |
89.95702972 |
98.43283087 |
96.75652 |
0.425 |
84.33781648 |
96.92542666 |
95.36736 |
0.25 |
75.27016773 |
95.10591673 |
94.69827 |
0.106 |
53.71744584 |
91.32744538 |
93.69783 |
0.075 |
47.93077259 |
90.1055847 |
93.29637 |
0.06 |
47.8 |
88.8 |
92.2 |
0.05 |
47.4 |
87.8 |
89.7 |
0.04 |
47 |
84.2 |
88.7 |
0.03 |
45.8 |
74.6 |
87.3 |
0.025 |
44.7 |
67.2 |
86.4 |
0.02 |
43.1 |
58.9 |
84.8 |
0.015 |
41.2 |
50.2 |
81.1 |
0.01 |
38.8 |
42.1 |
74.9 |
0.008 |
37.4 |
39 |
71.9 |
0.006 |
35.4 |
35.5 |
67.6 |
0.005 |
34.1 |
33.2 |
63.9 |
0.004 |
32.1 |
30 |
58.3 |
0.003 |
29.8 |
26.4 |
52.3 |
0.002 |
27.1 |
22.4 |
44.8 |
0.0015 |
25 |
19.6 |
40.1 |
0.001 |
22.9 |
17.2 |
31.7 |
8/24/2015 |
8/31/2015 |
|
|
|
||||
|---|---|---|---|---|---|---|---|---|
Sample |
Tare Weight (g) |
Wet Weight (g) |
Dry Weight (g) |
Water content (%) |
Tare Weight (g) |
Wet Weight (g) |
Dry Weight (g) |
Water content (%) |
A1 |
17.55 |
37.21 |
35.25 |
11 |
17.55 |
49.83 |
45.5 |
15 |
A2 |
17.1 |
39.74 |
37.49 |
11 |
17.1 |
42.17 |
38.59 |
17 |
A3 |
18.01 |
40.04 |
37.78 |
11 |
18.01 |
40.27 |
37.27 |
16 |
B1 |
17.71 |
45.32 |
40.01 |
24 |
17.71 |
42.16 |
37.09 |
26 |
B2 |
17.67 |
37.22 |
33.27 |
25 |
17.67 |
42.1 |
37.16 |
25 |
B3 |
17.97 |
43.95 |
38.89 |
24 |
17.97 |
49.74 |
43.55 |
24 |
C1 |
18.31 |
34.01 |
30.08 |
33 |
18.31 |
48.18 |
40.67 |
34 |
C2 |
17.75 |
38.12 |
33.39 |
30 |
17.75 |
48.48 |
41.09 |
32 |
C3 |
17.58 |
42.53 |
36.89 |
29 |
17.58 |
46.79 |
39.88 |
31 |
|
9/1/2015 |
|
|
9/2/2015 |
|
|
||
A1 |
17.55 |
52.89 |
45.83 |
25 |
17.55 |
55.32 |
47.57 |
26 |
A2 |
17.1 |
54.94 |
47.67 |
24 |
17.1 |
47.7 |
40.64 |
30 |
A3 |
18.01 |
55.05 |
47.32 |
26 |
18.01 |
56.13 |
48.25 |
26 |
B1 |
17.71 |
50.67 |
42.72 |
32 |
17.71 |
45.24 |
38.77 |
21 |
B2 |
17.67 |
44.87 |
38.78 |
29 |
17.67 |
40.18 |
34.06 |
37 |
B3 |
17.97 |
60.31 |
51.52 |
26 |
17.97 |
43.65 |
36.74 |
37 |
C1 |
18.31 |
49.31 |
40.64 |
39 |
18.31 |
42.72 |
35.47 |
42 |
C2 |
17.75 |
49.04 |
40.77 |
36 |
17.75 |
42.22 |
34.58 |
45 |
C3 |
17.58 |
47.36 |
39.48 |
36 |
17.58 |
45.87 |
36.42 |
50 |
|
9/7/2015 |
|
|
9/14/2015 |
|
|
||
A1 |
17.55 |
49.12 |
43.57 |
21 |
17.55 |
38.71 |
35.04 |
21 |
A2 |
17.1 |
46.79 |
41.52 |
22 |
17.1 |
45.07 |
39.91 |
23 |
A3 |
18.01 |
47.42 |
42.01 |
23 |
18.01 |
41.27 |
36.95 |
23 |
B1 |
17.71 |
39.18 |
34.67 |
27 |
17.71 |
39.08 |
34.78 |
25 |
B2 |
17.67 |
49.16 |
42.08 |
29 |
17.67 |
45.17 |
39.03 |
29 |
B3 |
17.97 |
49.17 |
42.92 |
25 |
17.97 |
37.8 |
33.8 |
25 |
C1 |
18.31 |
43.2 |
36.07 |
40 |
18.31 |
37.02 |
32.24 |
34 |
C2 |
17.75 |
39.14 |
32.64 |
44 |
17.75 |
36.95 |
32.16 |
33 |
C3 |
17.58 |
43.09 |
36.16 |
37 |
17.58 |
36.35 |
31.09 |
39 |
|
9/17/2015 |
|
|
9/22/2015 |
|
|
||
A1 |
17.55 |
42.83 |
37.92 |
24 |
17.55 |
47.8 |
42.55 |
21 |
A2 |
17.1 |
40.86 |
35.76 |
27 |
17.1 |
32.69 |
29.86 |
22 |
A3 |
18.01 |
44.14 |
39.36 |
22 |
18.01 |
45.62 |
41.02 |
20 |
B1 |
17.71 |
42.64 |
37.15 |
28 |
17.71 |
37.77 |
33.46 |
27 |
B2 |
17.67 |
35.19 |
30.98 |
32 |
17.67 |
46.37 |
40.06 |
28 |
B3 |
17.97 |
39.97 |
35.48 |
26 |
17.97 |
44.54 |
38.81 |
27 |
C1 |
18.31 |
38.37 |
32.07 |
46 |
18.31 |
44.77 |
37.19 |
40 |
C2 |
17.75 |
35.02 |
29.34 |
49 |
17.75 |
41.51 |
34.78 |
40 |
C3 |
17.58 |
32.77 |
27.71 |
50 |
17.58 |
41.65 |
36.25 |
29 |
|
9/28/2015 |
|
|
10/5/2015 |
|
|
||
A1 |
17.55 |
43.21 |
38.9 |
20 |
17.55 |
40.37 |
36.34 |
21 |
A2 |
17.1 |
39.34 |
35.41 |
21 |
17.1 |
37.88 |
36.27 |
8 |
A3 |
18.01 |
37.51 |
34.35 |
19 |
18.01 |
33.6 |
31.1 |
19 |
B1 |
17.71 |
35.24 |
31.04 |
32 |
17.71 |
41.62 |
36.05 |
30 |
B2 |
17.67 |
38.97 |
33.9 |
31 |
17.67 |
37.84 |
32.83 |
33 |
B3 |
17.97 |
39.15 |
34.08 |
31 |
17.97 |
37.88 |
32.9 |
33 |
C1 |
18.31 |
33.96 |
29.67 |
38 |
18.31 |
31.91 |
28.18 |
38 |
C2 |
17.75 |
38.08 |
32.13 |
41 |
17.75 |
34.99 |
29.78 |
43 |
C3 |
17.58 |
34.91 |
29.81 |
42 |
17.58 |
32.69 |
28.18 |
43 |
|
10/14/2015 |
|
|
10/21/2015 |
|
|
||
A1 |
17.55 |
31.5 |
29.4 |
18 |
17.55 |
37.42 |
34.04 |
20 |
A2 |
17.1 |
35.02 |
32.26 |
18 |
17.1 |
45.13 |
40.18 |
21 |
A3 |
18.01 |
31.16 |
29.16 |
18 |
18.01 |
32.68 |
30.21 |
20 |
B1 |
17.71 |
31.86 |
28.78 |
28 |
17.71 |
30.82 |
27.51 |
34 |
B2 |
17.67 |
33.13 |
29.66 |
29 |
17.67 |
27.68 |
25.34 |
31 |
B3 |
17.97 |
30.17 |
27.05 |
34 |
17.97 |
39.91 |
34.17 |
35 |
C1 |
18.31 |
29.53 |
26.46 |
38 |
18.31 |
34.2 |
29.58 |
41 |
C2 |
17.75 |
28.91 |
26.04 |
35 |
17.75 |
36.98 |
31.19 |
43 |
C3 |
17.58 |
31.06 |
27.31 |
39 |
17.58 |
30.41 |
26.52 |
44 |
|
11/26/2015 |
|
|
11/30/2015 |
|
|
||
A1 |
17.55 |
36.05 |
33.27 |
18 |
17.55 |
33.57 |
31.25 |
17 |
A2 |
17.1 |
43.92 |
35.81 |
43 |
17.1 |
32.14 |
29.59 |
20 |
A3 |
18.01 |
34.83 |
32.29 |
18 |
18.01 |
32.33 |
30.07 |
19 |
B1 |
17.71 |
28.3 |
26.37 |
22 |
17.71 |
33.92 |
30.78 |
24 |
B2 |
17.67 |
32.87 |
29.93 |
24 |
17.67 |
41.52 |
36.48 |
27 |
B3 |
17.97 |
29.98 |
27.5 |
26 |
17.97 |
31.51 |
28.52 |
28 |
C1 |
18.31 |
36.35 |
31.52 |
37 |
18.31 |
31.02 |
27.44 |
39 |
C2 |
17.75 |
34.61 |
30.46 |
33 |
17.75 |
31.31 |
27.66 |
37 |
C3 |
17.58 |
30.07 |
26.55 |
39 |
17.58 |
41.88 |
35.35 |
37 |
12/9/2015 |
||||||||
A1 |
17.55 |
42.95 |
39 |
18 |
||||
A2 |
17.1 |
28.55 |
26.73 |
19 |
||||
A3 |
18.01 |
30.48 |
28.44 |
20 |
||||
B1 |
17.71 |
37.09 |
34.18 |
18 |
||||
B2 |
17.67 |
38.64 |
34.66 |
23 |
||||
B3 |
17.97 |
31.4 |
28.47 |
28 |
||||
C1 |
18.31 |
32.94 |
29.05 |
36 |
||||
C2 |
17.75 |
35.89 |
31.01 |
37 |
||||
C3 |
17.58 |
28.48 |
25.52 |
37 |
||||
Supplementary Table 3 Water content monitoring
Supplementary Table 4 URL: Tuscany Region Weg GIS: Pedology Data Base, Landscape Units of study area.
The author declares there is no conflict of interest.

©2018 Brogna, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.