MOJ
eISSN: 2573-2919


Mini Review Volume 4 Issue 3
Instituto de Física, Universidade Federal do Rio de Janeiro, Brazil
Correspondence: ACF Santos, Instituto de Física, Universidade Federal do Rio de Janeiro, Rio de Janeiro, 21991-972, Brazil, Tel +5521 996279355
Received: May 27, 2019 | Published: June 10, 2019
Citation: Santos ACF. Revisiting the interaction of EUV photons and charged particles with dichloromethane and their role in the ozone depletion layer. MOJ Eco Environ Sci. 2019;4(3):123-126. DOI: 10.15406/mojes.2019.04.00143
Chlorine containing molecule, as the chlorofluorcarbons (CFCs) has long been acknowledged to cause the depletion of the ozone (O3) layer which safeguards the Earth from harmful solar radiation. CFCs can do it ascribed to the fact that they are chemically inert, having very long lifetimes in the atmosphere. Consequently, reach the stratosphere by chance where they can be broken down by ionizing and non-ionizing radiation, releasing chlorine atoms and ions and promoting the O3 layer depletion. This paper reviews the ionization and fragmentation of CH2Cl2 by extreme ultraviolet photons and fast protons.
The demand to understand the nature of the interaction of radiation with atoms and molecules is undeniable, since we inhabit a universe built from atomic building blocks. This world is formed by different regions with huge differences in composition, density, temperature, etc... A comprehension of macroscopic phenomena taking place in such world demands a corresponding comprehension of the mechanisms taking place at atomic level. Several macroscopic phenomena are driven by the atomic behavior. Thus, the study of the atomic and molecular level dynamics is fundamental.
Following the incidence and penetration into a material of ionizing radiation (particles or photons) from an external source, several phenomena take place as a direct consequence of a chain of events, the so-called radiation cascade. For instance, when the cosmic rays impinge on Earth or the sun shines on the Earth’s atmosphere, the inventory of the existing chemical species changes. In that case, the gases present in the atmosphere are ionized by photons or charged particles, giving rise to plasma.
Chlorine containing molecule, as the chlorofluorcarbons (CFCs) has long been acknowledged to cause the depletion of the ozone (O3) layer which safeguards the Earth from harmful solar radiation. CFCs can do it ascribed to the fact that they are chemically inert, having very long lifetimes in the atmosphere. Consequently, reach the stratosphere by chance where they can be broken down by ionizing and non-ionizing radiation, releasing chlorine atoms and ions and promoting the O3 layer depletion. One single Cl atom can break down several O3 molecules. This acknowledgment led in 1987 to the Montreal Protocol, which banned the production of CFCs by the industry.1–5
Nothwithstanding, shorter lived chemical compounds, such as CH2Cl2, have been shown to cause harm to ozone layer also.6 Albeit been largely applied as industrial solvent and in applications such as degreasing and dry-cleaning, CH2Cl2 had been assumed to break down very fast in the lower atmosphere and not reaching the stratosphere. The former view of the scientists has, accordingly, been that chemicals with short lifetimes did not represent harm to stratospheric ozone. Figure 1 shows the emission of halogen source gases to the atmosphere.
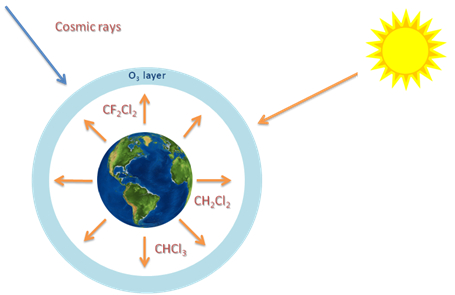
Figure 1 Emission of halogen source gases. Cosmic rays and solar radiation can interact with the gases in the atmosphere.
However, recently Hossaini et al.6 concluded, supported by observational data, that the potential for CH2Cl2 to cause damage the ozone budget is alarming. Dichloromethane has been identified as the most straightforward and used short-lived halogenic molecules, as a potential contributor to O3 layer depletion. Due to the fact that it is a cheap solvent, CH2Cl2 has been used in industry, Hossaini et al.6 reported that direct measurements of CH2Cl2 contribution in the Earth’s atmosphere to be rising fastly and to be disclosed into the stratosphere. After reaching the stratosphere, CH2Cl2 can be very effective in depleting the O3 layer.
The major external energy source for the Earth is the solar radiation. The radiant energy travels in the wave form at the speed of light c (299 792 458m/s, exactly, in vacuum). It is characterized by its wavelength l or by its frequencyn. They are related by the expression c=ln. Radiation is energy in transit. The energy propagates as subatomic particles of matter such as (electrons or protons, or as electromagnetic energy (photons). The solar electromagnetic radiation spectrum covers the entire electromagnetic spectrum from gamma and X-rays, to microwaves and radiowaves. Although, the most significant contribution to the electromagnetic spectrum associated with radiative energy transfer in the climate system ranges from the ultraviolet to near infrared, ionizing radiation, such as extreme ultraviolet (EUV) photons and cosmic rays,7,8 can cause great harm in the atmosphere.9–12 The majority of all energy that enters the Earth’s atmosphere has its origin in the Sun. The incident solar radiation can be absorbed, scattered, or reflected by the several gases present in the atmosphere. Ionizing radiation is a subatomic particle or photon sufficiently energetic (>10 eV) to directly or indirectly eject a target electron from an atom or molecule. Photons and charged particles both interact with matter via the electromagnetic fields they carry. Neutrons, as a neutral particle, do not ionize directly, but can ionize indirectly. After interacting with the nucleus of an atom or molecule, the neutron can either provoke emission of gamma-radiation due to the nuclear de-excitation, or break down the nucleus and eject charged particles, which can ionize directly the target.
Dichloromethane has been subject of several studies devoted to its ionization and fragmentation in both valence and inner shell.11,12 In this paper we review the data on the interactions of ionizing radiation (photons and protons) with the dichloromethane molecule.
In order to obtain experimental information on the molecular photofragmentation as a function of the photon and proton energy, experiments were performed at the Laboratório Nacional de Luz Síncrotron (LNLS) located in Campinas, São Paulo, Brazil and Laboratório de Colisões Atômicas e Moleculares (LACAM) located at the Physics Institute of Federal University of Rio de Janeiro, Brazil, respectively.
The photoionization of CH2Cl2 using synchrotron radiation was performed as follows: The molecular fragmentation studies were perfomed at the TGM (Toroidal Grating Monochromator) beamline. The time-of-flight mass spectrometry (TOF) is a widely used technique in molecular photo-fragmentation. The method, as will be described next, known as PEPICO, Photo-Electron-Photo-Ion Coincidence technique allows a very complete analysis of the fragmentation process, giving information about the fragmentation pathway, pattern, abundances, and dynamics of the photoionization. The fragments identification through the TOF is performed by the measurement of the charge/mass ratio. After the single or multiple ionization and fragmentation of the molecule induced by the photon, the positively ionized molecule or-fragment ions and the photoelectrons are extracted into opposite directions by a high electric field. The ejected electron collected by two micro-channel plates (MCPs) in chevron configuration, starts a time-to-digital converter (TDC), while, on the other side, cations, and drift in a field free region through the time-of-flight tube, are detected by MCPs, and stops the TDC. The fragment ions produced by the interaction with the linearly polarized light beam, are accelerated by a two-stage intense electric field extraction field. The coincidence experiments were performed with the TOF axis oriented linearly relative to the plane of polarization of the synchrotron light. The acquisition setup allows a multi-hit potentiality with 1 ns resolution.
The proton impact ionization and fragmentation of CH2Cl2 was performed as follows: the experimental apparatus and methods employed in these measurements were the same as those described in.9 Thus, only a brief summary will be presented here. Proton beams were produced from H- beams, delivered by a cesium sputtering source. The beams were accelerated by the 1.7MV tandem accelerator at the Federal University of Rio de Janeiro (UFRJ). The H- beam crosses the nitrogen cell stripper. The emergent p+ beam is directed through a bending magnet into the beamline, with base pressure around 10−8 Torr. The p+ crosses a effusive gas jet of CH2Cl2 and is detected by a surface barrier detector located at the end of the beamline. Another TOF spectrometer is used to analyze the charged fragments generated in the gas jet by the proton beam.
Photoionization takes place when an ionizing photon (Energy >10eV) is absorbed (photoabsoption) in the process of ionizing an atomic or molecular electron. This is the photoelectric effect interpreted by Albert Einstein. Photoabsorption by a free electron is forbidden process because it cannot conservate energy and momentum simultaneously. Photons and charged particles both interact with matter via the electromagnetic fields they bring (virtual photons in the case of charged particles).
The thermosphere is the layer in the atmosphere situated between the mesosphere and the exosphere. Within this layer, ionizing radiation provokes photoionization and/or photodissociation of atmospheric molecules, producing ions and changing the chemical inventory in the ionosphere.
Figure 2 shows the mass spectra of CH2Cl2 after photon absorption at 20 eV. The main fragmentation channels are:
(33 %) (i)
(67 %) (ii)
Figure 3 shows the branching ratios of the fragments of the CH2Cl2 molecule after 20eV photoabsorption.4 The fragmentation channel leading to tge CH2Cl++Cl dominates (67 %) at 20eV photon impact.
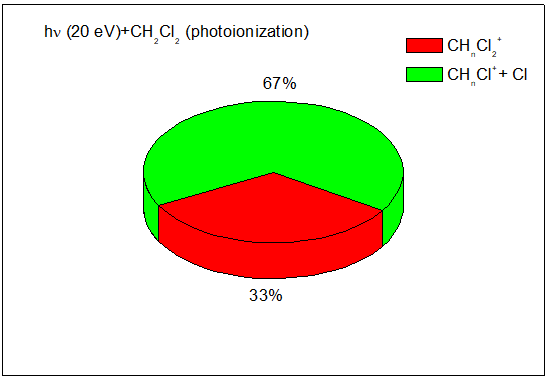
Figure 3 Branching ratios of the fragments of the CH2Cl2 molecule after 20eV photoabsorption.4
Earth is regularly exposed to high-energy charged particles from cosmic rays. Cosmic ray particles lose kinetic energy mainly by ionizing electrons from the targets with which they collide in the atmosphere. The extraterrestrial composition of the cosmic rays is 98% protons and heavier charged nuclei and 2% electrons and positrons. The nuclei composition of cosmic rays, in the energy range 100MeV/amu to 10GeV/amu, is 87% protons, 12% alpha particles, and 1% heavier nuclei. The evidence of the correlation between cosmic rays, chlorofluorocarbon (CFC) dissociation, and O3 layer depletion was found from satellite data.7,8
Alcantara et al.6 performed a study for the fragmentation of the CH2Cl2 molecule for collisions with 0.2–2.0MeV p+ beam. Ion yields for fragmentation products have been measured as a function of the projectile velocity. Their results have shown that the measured fragments were associated to the release of a chlorine atom. By combining the information from the molecular orbital energies, the authors have estimated the relative contributions of the molecular orbitals to the total ionization of CH2Cl2 by proton impact. Figure 4 shows the mass spectra of CH2Cl2 after proton impact at 400keV. At 400keV, the main fragmentation channels are:
(27 %) (iii)
(48 %) (iv)
(12 %) (v)
Figure 5 shows a pie chart of the main fragmentation products after ionization and fragmentation of CH2Cl2 by 2.0MeV protons from ref.9
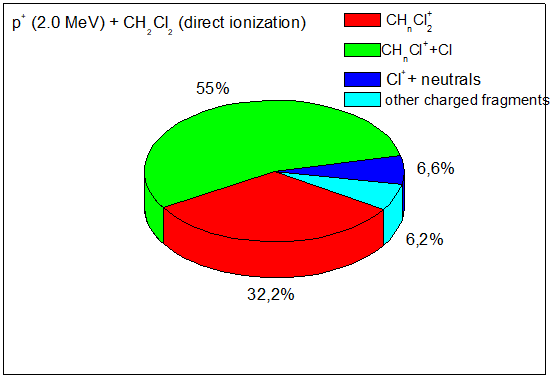
Figure 5 Branching ratios of the main fragments of the CH2Cl2 molecule after 2.0MeV direct ionization by proton impact.9
In this paper a reviews of the ionization and fragmentation of CH2Cl2 by extreme ultraviolet photons and fast protons was presented. An impressing point about the ozone hole in the Earth’s atmosphere is that notwithstanding the Montreal protocol, the recovery rate of the ozone layer has been observed to be less than forecasted. CH2Cl2 has been identified as a dangerous threat to Earth’s atmosphere.
None.
The authors declared there is no conflict of interest.

©2019 Santos. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.