MOJ
eISSN: 2573-2919


Mini Review Volume 5 Issue 3
1Department of Systems Engineering and Plant Safety, Institute of Instrumental and Environmental Technology, Otto von Guericke Universität, Germany
2Regional Centre for Energy and Environmental Sustainability, University of Energy and Natural Resources, Ghana
Correspondence: KO Ansah Amano, Otto von Guericke Universität, Institute of Instrumental and Environmental Technology, Department of Systems Engineering and Plant Safety, 39106, Magdeburg, Germany, Tel +15213037071, Fax +493916711128
Received: May 14, 2020 | Published: June 19, 2020
Citation: Amano KOA, Ntiri-Asiedu AG. Mercury emission from the aluminium industry: a review. MOJ Eco Environ Sci. 2020;5(3):129-135. DOI: 10.15406/mojes.2020.05.00185
There has been a rising global concern of mercury because of its persistent nature, long-range transport and toxicity. Mercury possesses serious health effects on living organisms and the environment. Mercury, mostly in inorganic form, is present in almost all categories of rocks. It may be released through natural occurrences and/or anthropogenic activities such as aluminum production. Stable organic mercury compounds, for example methyl mercury (CH3Hg), are formed by the attachment of mercury to one or two carbon atoms. Depending on the source of bauxite, a substantial amount of elemental mercury is released by aluminum industries. Emitted elemental mercury contributes to increasing global atmospheric reserve of mercury hence decreasing mercury emissions plays a key role in lowering the contribution of anthropogenic activities to the global atmospheric mercury budget. In general, all three forms of mercury (elemental, inorganic and organic mercury) have the potential of causing adverse health effects at sufficiently high doses. Mercury emissions are readily absorbed through the alveoli membranes and gastrointestinal tract affecting other systems. Fetuses and individuals often exposed to mercury (chronic exposure) are two classes of people who are more susceptible to harm caused by mercury. This paper discusses mercury metal and oxide emission from the aluminum industry.
Keywords: aluminum, anthropogenic activities, bauxite, bioaccumulation, metal mercury
Possessing serious adverse effects on living organisms, and the environment, there has been a rise of global concern on the presence of mercury compounds. This is because of the persistent nature, long-range transport, and high toxicity of most mercury compounds.1 Mercury has a high potential of bioaccumulation through the air, food, and drinking water giving long term detrimental effects on the lungs, neurological, digestive and immune systems.2 Chemically, mercury exists in three forms namely elemental (or metallic, Hg0) mercury, inorganic mercury compounds, and organic mercury compounds. At room temperature, elemental mercury exists as a liquid and it is used in some thermometers, fluorescent light bulbs and various industrial processes. It is also used for dentistry and consists of about 50 % of amalgams.3 If metallic mercury is not enclosed at room temperature, some amount of it will evaporate to form mercury vapours. Mercury vapours are colorless and odorless. Also, when mercury (Hg+, mercurous or mercury I, and Hg2+, mercuric or mercury II) combines with elements such as chlorine, sulphur or oxygen, it produces inorganic compounds in the form of salts.4 As shown in Figure 1, some of the salts produced from the combination of such elements are mercury sulphide (HgS, purified from cinnabar), mercurous chloride (Hg2Cl2, also named as calomel), and mercuric chloride (HgCl2). Mercury, mostly in inorganic form, exists in all categories of rocks including bauxite, limestone, shales, sandstone, basalt, and others. Furthermore, stable organic metallic compounds such as methyl mercury (CH3Hg), dimethyl mercury (CH3)2Hg) and ethyl mercury (C2H5Hg) are formed by the attachment of mercury to one or two carbon atoms.
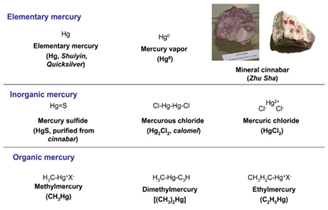
Figure 1 Chemical structure of some mercury compounds.4
Mercury is an atmospheric pollutant with a complex biogeochemical cycle. The atmospheric cycling comprises chemical oxidation/reduction in both gaseous and aqueous states, deposition and release from natural surfaces as well as emissions from both natural and anthropogenic sources.5 Even though mercury can be released through natural occurrences such as volcanic eruption and weathering of rocks, a significant amount of studies show that anthropogenic activities can initiate its dispersion into the atmosphere, water and soil using complex transport and chemical transformation.6 Anthropogenic activities occurring in numerous industrial processes such as aluminum production, hazardous waste incineration, coal combustion, incineration, gold mining, and certain chemical manufacturing operations, do increase mercury emission into the environment.7 An estimate of approximately 2220 metric tons of mercury is emitted worldwide each year from anthropogenic sources.8 Studies indicate about 50–75% of atmospheric mercury is released in the United States as a result of anthropogenic activities.5 The principal source of mercury emissions in sectors involving the manufacture of non- ferrous metals including aluminum, cement, coal or oil and production of pig iron and steel.9 As shown in Table 1, a study in 2005 indicates highest global emission of mercury occurs from the combustion of fossil fuels followed by cement production and production of non-ferrous metals such as aluminum.10 Additional studies by Pirrone et al signifies, China released a greater share of global atmospheric mercury emission between the periods of 2003-2006 as shown in Table 2.
Region |
Stationary combustion |
Non-ferrous metals production |
Pig iron and steel production |
Cement production |
Gold production |
Mercury Production |
Waste Incineration |
Caustic Soda Production |
Other Sources |
Total |
Africa |
37.3 |
2.1 |
1.6 |
10.9 |
8.9 |
0 |
0.6 |
0.1 |
0 |
61.6 |
Asia (Excluding Russia) |
622 |
90 |
24.1 |
138 |
58.9 |
8.8 |
5.7 |
28.7 |
0.6 |
977 |
Europe (Excluding Russia) |
76.6 |
18.7 |
18.8 |
0 |
0 |
10.1 |
6.3 |
14.7 |
145 |
|
North America |
71.2 |
5.7 |
14.4 |
10.9 |
12.9 |
0 |
15.1 |
6.5 |
7.2 |
144 |
Oceania |
19 |
6.1 |
0.8 |
0.4 |
10.1 |
0 |
0 |
0.2 |
0 |
36.6 |
Russia |
46 |
5.2 |
2.6 |
3.9 |
4.3 |
0 |
3.5 |
2.8 |
1.5 |
69.8 |
South America |
8 |
13.6 |
1.8 |
6.4 |
16.2 |
0 |
0 |
2.2 |
1.5 |
49.6 |
World |
880 |
141 |
45.4 |
189 |
111 |
8.8 |
35.5 |
46.8 |
25.5 |
1480 |
Table 1 Global emission of mercury by-product from anthropogenic sources (tonnes).10
|
Region |
SCa |
NFMP |
PISP |
CP |
CSP |
MP |
GP |
WD |
OP |
T |
Reference year |
|
Australia1 |
2.2 |
11.6 |
0.8 |
0.9 |
0 |
0 |
0.3 |
0.2 |
0.6 |
16.6 |
2005 |
|
S. Africa2 |
32.6 |
0.3 |
1.3 |
3.8 |
0 |
0 |
0.3 |
0.6 |
1.3 |
40.2 |
2004 |
|
S. America3 |
8 |
13.6 |
1.8 |
6.4 |
2.2 |
0 |
16.2 |
0 |
1.5 |
49.7 |
2005 |
|
Russia3 |
46 |
5.2 |
2.6 |
3.9 |
3.9 |
0 |
4.3 |
3.5 |
1.5 |
69.8 |
2005 |
|
Europe3 |
76.6 |
18.7 |
0 |
18.8 |
6.3 |
0 |
0 |
10.1 |
14.7 |
145.2 |
2005 |
|
N. America4 |
65.2 |
34.7 |
12.8 |
15.1 |
15.1 |
0 |
0 |
13 |
1.7 |
152.8 |
2005 |
|
India5 |
124.6 |
15.5 |
4.6 |
4.7 |
6.2 |
0 |
0.5 |
77.4 |
7.5 |
240.9 |
2004 |
|
China2 |
268 |
203.3 |
8.9 |
35 |
0 |
27.5 |
44.7 |
14.1 |
7.6 |
609.1 |
2003 |
|
Total |
623.2 |
302.9 |
32.8 |
88.6 |
27.8 |
27.5 |
66.3 |
118.9 |
36.4 |
1324.3 |
|
|
Rest of the world6 |
186.8 |
7.1 |
10.4 |
147.1 |
135.1 |
22.5 |
333.7 |
68.5 |
28.2 |
989.4 |
2006 |
|
Total |
810 |
310 |
43.2 |
235.7 |
162.9 |
50 |
400 |
187.4 |
64.6 |
2313.7 |
|
Table 2 Global emissions of total mercury from major anthropogenic sources (Mg .yr−1).7
Mg, mega grams(
1 tonne); SC, stationary combustion; NFMP, non-ferrous metal production; PISP, pig iron and steel production; CP, cement production; CSP, caustic soda production; MP, mercury production; GP17, gold production; WD, waste disposal; OP, other processes; T, total
References: (1)11; (2)12; (3)10, (4)13–15; (5)16; (6)17
Presently, mercury concentration in air has been identified to be of the range 2-10ng/m3 with the level of mercury in drinking-water found to be nearly equivalent to that of rain, with an average of approximately 25ng/L and the average ingestion of mercury from food is in the range 2–20μg/day.18 Interestingly, just about 7–15% of consumed mercury in food is absorbed into the human body.19 Mercury emissions are readily absorbed through the alveoli membranes and gastrointestinal tract affecting other systems. Exposure at maximum contaminant level (MCL) for an extended period causes serious health consequences.20 Aluminum production has been identified as a major source of mercury emission from non-ferrous process. This paper discusses mercury metal and oxide(s) emission from the aluminum industry and its harmful effects.
Aluminum is known to be the third most abundant element in the earth’s crust.21 Due to the physical and chemical properties of aluminum it has found its application in transportation, construction, electrical and consumer goods. Production of primary aluminum involves two independent energy-intensive processes to transform the ore, which is bauxite, to the metal by electrolytic reduction.22 Bauxite resources are present in various parts of the world; between 55 and 75 billion tons were estimated in 2017. It is more abundant in tropical regions in Africa such as Guinea (32%), Oceania (23%), South America and the Caribbean (21%), Asia (18%).23,24 Bauxite is made up of approximately 45-60% aluminum oxide, combined with a number of impurities such as sand, iron, and other metals. The Bayer process is initially used to refine bauxite ore to obtain aluminum oxide. Afterwards, the Hall-Heroult process is used to smelt the produced aluminum oxide to release pure aluminum.25 There are trace amounts of mercury (0.02–1.5mg/kg) found in bauxite.26 However, due to the huge amount processed in Bayer refining, mercury accumulate in the resulting Bayer liquor, aluminum hydrate, and oxalate.27 Generally, mercury is produced in the form elemental mercury vapor mainly in the upstream section of the refining process.28 A greater fraction of inorganic mercury compounds produced is converted into elemental mercury at temperatures higher than 700 to 800˚C, because they become thermally unstable at such higher temperatures.29,30 Upon reaching the stack where the temperatures are lower, the elemental mercury reacts with other constituents such as oxygen or chlorine present in the emissions to form inorganic compounds.31 Studies from ALCOA Bayer refineries show mercury emissions into air from Bayer refineries are almost 100 % elemental mercury.32 There is a substantial difference in mercury concentration depending on the source of bauxite. Findings by Dobbs et al. also indicate forms of mercury produced vary according to bauxite sources by selective extraction of mercury.27 Again, mercury emissions into air and mercury loss to residual bauxite demonstrate that release of mercury can vary significantly from plant to plant. This is because, mostly, mercury is released by some refineries from calcinations whereas other plants’ emissions are from digestion processes. UNEP/AMAP’s statistical data presented in 2012 signifies mercury released from the production of aluminum ranges from 2.1–11.6mg/kg with an average amount of 5.9mg/kg contributing to 0.3% of global emission.
Aluminum production
For aluminum’s wide use and application in areas such as transportation, building and construction, health, packaging etc., its production keeps increasing as the years come by. The first aluminum production was made during in the 18th Century.33 aluminum production involves three major processes which are mining of the aluminum ore (bauxite), extraction of alumina (Al2O3) by Bayer process and electrolytic reduction of alumina into metallic aluminum through Hall-Héroult process.33 The Bauxite is mined by a method known as open cut mining. This method involves excavation of bauxite at the surface of the reserve site. The sky is viewable from the mine site. Excavated ore is transported to the plant site for milling and further processing. The next step is refinery of alumina from the bauxite through the Bayer Process. This involves the digestion of milled bauxite in a concentrated solution of caustic soda (NaOH) and sodium aluminates (NaAl(OH)4) at an elevated temperature (220-260˚C) and pressure.34 Other researchers report bauxite is heated in a concentrated solution of caustic soda (sodium hydroxide: NaOH) and sodium aluminates temperatures between 140˚C and 150˚C for gibbsitic bauxite and between 220˚C and 270˚C for boehmitic and diasporic bauxite, under high pressure in the Bayer process.35 After a given period of time, the bauxite residue known as red mud (due to its colour and nature) which contains metal oxides is allowed to settle out and separated from the aluminum-containing liquor (green) using filters.36 To reduce production cost, it is a common practice to thicken and wash the red mud to recover as much NaOH as possible for re-use.36 The aluminum-containing solution is then precipitated to form the crystallized aluminum trihydroxide (Al(OH)3) simply known as hydrate. The hydrate is calcined in a kiln at a temperature greater than 1000˚C to form alumina (Al2O3).35 This produced alumina is then used for aluminum production. This stage involves electrolytic reduction of alumina by Hall-Héroult process. Generally, in the Hall-Héroult process the produced alumina is dissolved in a molten cryolite (Na3AlF6) bath at 1000˚C from which it is deposited by applying low voltage high electric current across carbon electrodes.37 At the cathode, molten aluminum is released while CO2 is formed from the reaction between oxygen and carbon at the anode.25
Gaseous mercury compounds are released in the digestion section of Bayer process and during the electrolytic reduction of alumina through Hall-Héroult process. The gaseous emissions from these sections are treated to reduce their mercury concentrations to an allowable limit or completely remove them if possible. The resulting gas is subsequently released from refinery vents and stacks together with non-condensable gases. The mercury compounds released along are noted to be harmful and encounters could have effect on the human body.
A case study of aluminum Company of America (ALCOA) plant mercury emission
The aluminum Company of America (ALCOA) is a key player in the aluminum processing venture.38 Table 3 shows their annual production of bauxite, alumina and aluminum from the period of 2016 to 2018. Due to the historic involvement of ALCOA in research and development, they have made achievementson the improvement of the aluminum production process. As depicted in Figure 2, was a significant reduction of mercury emissions from 0.2g per ton of alumina produced to 0.12g per ton of alumina produced (a clear 40% decrease) between 2013 and 2018.38 At ALCOA's three Western Australian refineries, personal sampling for mercury among refinery employees in a range of roles and operating areas consistently gave results to be within the then current 2013 American Conference of Governmental Industrial Hygienists (ACGIH) Threshold Limit Value (TLV) of 0.025mg/m3.28

Figure 2 Mercury Emissions by AWAC.39
Product |
Annual production capacity (million dry metric tons ) |
||
2016 |
2017 |
2018 |
|
Bauxite |
45 |
45.8 |
45.8 |
Alumina |
15.1 |
13.1 |
12.9 |
aluminum |
3.1 |
2.3 |
2.3 |
Table 3 ALCOA’s annual production capacity (ALCOA Annual Report 2016, 2017, 2018)
Elemental and mercury oxide emission by the aluminum industry
Generally, predominant amount of elemental mercury is released during aluminum production process.32,40 A report of the Pinjarra Refinery indicates approximately 430kg of mercury is released each year due to the high throughput of the plant.41 Mercury concentration in the atmosphere is influenced by the degree of volatility of its compound at ambient condition. Elemental mercury (Hg0) is considered to have much higher levels of vapor pressure than mercury oxide (HgO).42 Primarily, oxidation reaction of elemental mercury and ozone produces oxidized divalent mercury (II) ion (Hg2+) which combines with oxygen to form mercuric oxide (HgO).43 Ozone (O3) in the troposphere is a secondary air pollutant produced from photochemical reactions.23 Owing to oxidation of elemental mercury by ozone, mercuric oxide produced possesses short residence time ranging from hours to days..44
More so, substantial amount of elemental mercury from aluminum production is washed out of the atmosphere by hydroxyl radical reaction. Hydroxyl radical in the atmosphere is generated by the reaction of water vapor with atomic oxygen present in ozone, nitrogen oxides (NOx) and hydrogen peroxide (H2O2).45 Similarly coal-fired power plants which aluminum industries employ enhance oxidation of elemental mercury in selective catalytic reduction (SCR) unit followed by the absorption of the oxidized mercury in a wet scrubber as an economic means of reducing mercury emission.46 The amount of mercury emitted is known to pose minimum risk to the immediate environment because elemental mercury has residence time of 1–1.5years.27 Emitted elemental mercury increases the global atmospheric reserve of mercury. Hence, decreasing mercury emissions from aluminum production plays a key role in lowering the contribution of anthropogenic activities to the global atmospheric mercury budget.
Health effect of mercury emission
In general, all three forms of mercury (elemental, inorganic and organic mercury) have the potential of causing adverse health effects at sufficiently high doses. Mercury is known to be transported into the environment by air and water, as well as by biological organisms through the food-chain. In the environment, elemental mercury may combine with chlorine, sulphur, and other elements to form inorganic compounds. Most common forms of these inorganic mercury compounds are mercury sulphide, mercuric chloride and mercurous chloride. Mercury (in the form of elemental or metallic mercury) when released through aluminum production into the environment can be converted by bacteria in its form as inorganic mercury to produce organic mercury compounds. Bacteria transforms greater fraction of inorganic mercury mainly into methyl mercury.47 Another form of organic mercury is ethyl mercury derived from the metabolism of thimerosal. Methyl mercury is very distinct to ethyl mercury. Ethyl mercury is easily digested by the human body and does not accumulate. In the pharmaceutical field, ethyl mercury is used as preservative in the manufacture of some vaccines and does not have any known health risk.48 Meanwhile, methyl mercury is not easily broken down, hence, its bioaccumulation.
Routes of exposure to mercury emissions
The main exposure route is through inhalation of mercury contaminated vapour, ingestion and dermal contact during the production process. Approximately, 80% of the total elemental mercury is absorbed through inhalation of the contaminated air in and around processing plants.49 Absorption of inorganic mercury through inhalation route is much lower than through ingestion.50 No human studies have been found from which the absorption of inhaled inorganic mercuric compounds might be estimated. As with respect to any inhaled aerosol, absorption would be estimated primarily by the size and solubility of the particles and absorption media or surface. In a study with dogs, using a mercuric oxide aerosol with a median particle diameter of 0.16μm, the absorption was estimated to be approximately 45%.51
On the contrary, less than 0.01 percent of the ingested elemental mercury is poorly absorbed from the gastrointestinal tract.52 Exposure to the inorganic mercury occurs through the ingestion by lower organisms present in polluted soil or water which may then be transferred to plants or animals that feed on them. Soil polluted by mercury or the redistribution of polluted water has the possibility to enter the food chain through plant and livestock. Approximately 7-15% of inorganic mercury compounds are absorbed through the intestine after ingestion.52 Ingestion of fish and other foods contaminated with methyl mercury leads to the absorption of about 95% of methyl mercury from the gastrointestinal tract.53 Dermal exposure of mercury vapors is minimal and it accounts for only 2.6% of the total elemental mercury absorption.30 Exposure to the inorganic mercury compounds arises from the dermal exposure while handling contaminated soil and water and only 2-3% of the total is absorbed.54
Effect of exposure to mercury emission
Generally, occupational risk occurs due to inhalation of elemental and/or inorganic mercury. Most often, occupational mercury exposures occur when workers inhale elemental mercury vapours. Some dermal absorption may arise from skin contact with polluted air from the aluminum processing plant, but the extent is low (less than 3% of the inhaled dose).55 Elemental mercury has the potential of being readily absorbed through the placental barrier (transplacental absorption) increasing the risk of foetus being exposed to mercury from the pregnant woman’s body.56 Meanwhile, occupational exposure to inorganic mercury compound of a dose higher than a general threshold limit value (TLV) of 0.025mg/m3 causes some dangerous effect on the human body.57 These aforementioned species of mercury can be absorbed into the body by inhalation of its aerosol generated during aluminum production, through the skin and by ingestion. Prolonged exposure might cause skin sensitization. Some vital body parts such as the central nervous system, peripheral nervous system and kidneys may be impaired due to chronic exposure above the TLV. In the meantime, acute oral exposure to inorganic mercury bares relatively greater acute health effects than elemental mercury.52 Deaths resulting from oral injection of inorganic mercury have been attributed to renal failure, cardiovascular collapse, and severe gastrointestinal damage. For instance, lethal doses of mercuric chloride ranges from 10 to >50mg mercury/kg body weight.55
On a global basis, 89 % of Hg which end up being deposited in aquatic systems is re-emitted to the atmosphere, mainly in the form of Hg0 and also in traces as (CH3)2Hg.58 Through bioaccumulation, aquatic organisms which exist close to the sea or ocean bed such as marlin, tuna, shark, swordfish, king mackerel and tilefish have high concentration of methyl mercury.59 Analysis of deep ocean waters showed mercury concentration ranging from 0.7 to 2pM (picomolar, 1pM=10-12mol/L).60,61 Methyl mercury is attached to fish protein when absorbed through consumption of contaminated food or through gills. In some instances, methyl mercury concentration of carnivorous fish such as freshwater bass, walleye and pike, and marine shark and swordfish in the nearby vicinity of production plants, bioaccumulated to about million times higher than in surrounding water.62 Even though fish seem to be tolerant to high level of methyl mercury, there have been circumstances of fatalities caused by severe poisoning. Typical example is the Minamata disease (MD) which was discovered officially in May 1956. The marine produce from Minamata Bay showed high concentration of methyl mercury contamination ranging from 5.61 to 35.7ppm.63 High concentrations (max. 705ppm) of the contaminants was detected in the hair of patients from Shiranui Sea coastline. This indicates that, improper treatment of waste/tailing from manufacturing processes such as the aluminum industry poses a threat to the environment.
Similarly, recent studies indicate crops such as rice grown in mineral ore mining and processing areas such as bauxite mining sites could be the main pathway of methyl mercury exposure to humans. This is a major concern since most aluminum production plants are sited closed to the source of raw material, bauxite. Some agricultural produce in such mining areas tend to absorb some quantities on mercury. In premature rice plants, large amount of methyl mercury is found in the leaf and stalk with greater portion transferred to seed during the harvest period.64
Measures to reduce the mercury emissions
Reducing the rate of mercury emission from the aluminum industry is very necessary to minimize local as well as global deposition of mercury. Required measures must be taken to reduce the emission of mercury compounds in order to avoid the possible effects on fish, wildlife and people. The following measures could be taken to protect people and the environment from harm induced by mercury emissions.
Activated carbon injection
Activated carbon injection is used to increase the concentration of particulate carbon in flue gas. In this method, dry-powdered or wet-slurried carbon is injected into the flue gas prior to the air pollution control equipment.65 The adsorption of mercury compounds on the surface of activated carbon depends on the exhaust stream temperature, thus obeying Langmuir adsorption model.66 An inverse relationship exists between the bag house temperature which is approximately 120 to 2000C and the reduction of mercury emissions by the activated carbon injection.67 The particle size of the activated carbon also plays a major role on the rate of absorption of mercury.There is a correlation between the average removal of mercury across the air pollution control with the carbon injection rate. A study indicates that, at carbon feed rates of approximately 100mg/m3, mercury removal across the air pollution control equipment increases beyond 90%.65 Plants which employ the use of carbon injection showed a reduction of 70 to 90% of mercury emissions as against other plants that showed a reduction of 30 to 65 % of emissions.30 Several authors have proposed that in addition to adsorbing Hg (II) compounds, activated carbon particulates may serve as a catalyst for the oxidation of elemental mercury (Hg°) to Hg(II) in the exhaust stream, thus facilitating the removal of elemental mercury (Hg°).30 Other investigation also indicates powdered virgin activated carbon has 91% and 98 % mercury removal under batch and flow conditions respectively.40
Wet lime/limestone flue gas desulfurization
The use of Wet lime/limestone flue gas desulfurization procedure has been shown to remove between 8 and 72% (average 52%) of mercury from the flue gas.68 As a result of its high water solubility, divalent mercury can be easily removed, thus the wet systems remove principally the oxidized elemental mercury. Interestingly, under pH greater than three (pH>3) and chloride ion concentration less than 0.1 Molar (C1-<0.1M), the sulphur dioxide (SO2) present in the flue gas affects the reduction followed by subsequent removal of divalent mercury. This leads to the formation of elemental mercury vapours that escape in the exhaust stream.69
Sodium tetrasulfide/sodium sulphide injection
Sodium tetrasulfide (Na2S4) reacts with mercury in flue gas to form solid mercury (II) sulphide (HgS) which can then be captured by particulate control equipment. This procedure finds its application in the removal of both elemental and ionic mercury compounds. Mercury (II) sulphide commonly known as cinnabar is known to be thermally stable and insoluble. Therefore, the process effectively immobilizes the mercury by chemical binding to produce mercury sulphide which is then removed from flue gas by particulate control equipment such as a bag house or an electrostatic precipitator (ESP). Mercury reduction rates with Na2S4 injection could increase to about 90%.70 Similarly, Sodium sulphide (Na2S) undergoes a chemical reaction with mercury present in flue gas to produce solid HgS which could also be captured by particulate control equipment.65 Mercury reduction rates with Na2S injection range from 73 to 99%, although poorer capture efficiencies have been reported, possibly due to difficulty with removal of much finer HgS particulates formed.71
Generally, aluminum production is considered as one of the major sources of mercury emission by anthropogenic activities. Implementation of independent energy-intensive processes (Bayer and Hall-Heroult processes) in the manufacture of aluminum produces some amount of mercury. Recent studies have shown high mercury concentration in the form of elemental mercury in the upstream section of the aluminum refining section. This adds up to the global mercury budget when improperly managed.
Specifically, two classes of people are more susceptible to the harm from increasing concentration of mercury produced through anthropogenic processes such as aluminum production. Fetuses are most vulnerable to developing defects at the formation phase. The second class is people frequently exposed (chronic exposure) to high levels of mercury. Majority of these people are in the processing industries such as aluminum production industry and the fishing sector where they are highly prone to mercury exposure above biological working value.72
Exposure to mercury can be checked in with numerous approaches. One of the approaches that can be employed is the use of extremely strict measures developed by global policy makers to control global mercury emission by industrial activities. In industries such as aluminum production firms where mercury cannot be completely eliminated, safer work ethics are required to reduce exposure. The production plant has to be properly designed to prevent leakage into the processing area.
In most modern aluminum process plants, mercury is oxidized within downstream equipment before it is released through a stack into the atmosphere. The most popular technology entails injection of an absorbent and activated carbon into the exhaust stream of the plant. In humans, dietary organic selenium has been proven to eliminate elemental mercury.73 Research has identified dietary selenium to be very active with acceleration of the excretion and demethylation of MeHg from employees with high risk of occupational exposure.74 This prevents bioaccumulation and toxicity of methyl mercury.
None.
None.
The authors declare there are no conflicts of interest.

©2020 Amano, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.