MOJ
eISSN: 2573-2919


Research Article Volume 3 Issue 4
1Al-Faraby Kazakh National University, Republic of Kazakhstan
2Institute of Evolution, University of Haifa, Israel
3Institute of Botany and Phytointroduction, Kazakhstan
Correspondence: Barinova S, Institute of Evolution, University of Haifa, Mount Carmel, 199 Abba Khoushi Ave, Haifa 3498838, Israel
Received: July 19, 2017 | Published: August 2, 2018
Citation: Jiyenbekov A, Barinova S, Bigaliev A. Algal comparative floristic of the alakol lake natural state reserve and other lakes in Kazakhstan. MOJ Eco Environ Sci. 2018;3(4):252-258. DOI: 10.15406/mojes.2018.03.00096
Comparative floristic analysis was doing for the new data about algal diversity of the Alakol Lake and 42 algal floras of the Kazakhstan lakes studied by us earlier in purpose to reveal major environmental factors that influenced algal floras forming process in arid region of Eurasia. Algal diversity was studied in first time during 2015– 2017. We revealed 208 taxa of species and infraspecies level from five taxonomic Divisions. Diatom algae were prevailing in species richness of all studied areas communities excluding charophyte communities in the northern part of the lake. In present level of the Kazakhstan lakes floras study, the Alakol Lake flora is one of ten richest, and therefore can be included to the Kazakhstan floras analysis. Comparative floristic analysis with statistical programs helps us to reveal that floristic tree of similarity is better to use for the lake size and salinity class relation on algal diversity. We reveal that algal diversity is higher in the large lakes like Alakol, Balkhash and Shardara as well as in the lakes with low saline fresh water. Comparison of studied floras with inclusion– crossing dendrites statistical generation reveals not only influence of the lake size but also salinity class for total diversity and diversity of diatoms. For total diversity, Chlorophyta and diatoms species richness the floristic core was define the special group of mountain lakes. Cyanobacteria species richness was higher in fresh lakes class salinity 1, then in the lakes of 2–3 salinity class. We can to conclude in this calculation base that salinity and the size of the lake play the major role for species richness forming process in the Kazakhstan lakes algal flora. Comparative floristic analysis revealed the tendencies in forming process of the Kazakhstan algal diversity, which is under influence of arid climatic factors such as high evaporation and salinity of the lakes increasing.
Keywords: algae, lakes, flora, comparative floristic, Kazakhstan
The Alakol Lake is a unique natural water body with a gradient of salinity in which algal diversity has been never studied before our research. The lake received status Alakol Lake State Natural Reserve from 1998 in the frame of the Convention on Biological Diversity ratified by the Parliament of Kazakhstan in 2004, thus affirming its desire to preserve the unique richness of nature in the country. In the first step of investigation of the new natural reserve monitoring was the identification of the biological diversity. Whereas some aquatic invertebrates were studied accidentally in the Alakol Lake,1 the algal diversity study is in initial step because up o current research the information about algal species of the Alakol Lake was absent. As a result of our preliminary study, we revealed 203 algal species (208 with infraspecific taxa) from five taxonomic Division during 2015–2017 sampling period.2 This research project is continue our algal floristic study that started in 2000 with support of WWF on the territory of Kazakhstan.3–7. So, the Alakol Lake algal flora represent next step of floristic study of water bodies in protected areas of Kazakhstan. In Kazakhstan, the arid and semiarid dry grasslands to deserts are widespread in the upper reaches of Ob River Basin and the Turkestan Desert.8 Large highly mineralized lakes Balkhash, Tengiz, Issyk Kul and Karakul, as well as the Aralian and Caspian seas, are confined to this climatic region.9 Phytogeographically, this region is situated near the boundary of the Irano– Turanian province and the Boreal province north of it.10 A large number of lakes in this area are protected on account of their importance for biodiversity conservation.11 The aim of present work is to compare algal floristic diversity in the protected lakes of Kazakhstan, including the Alakol Lake with algal floras of other large water bodies such as Balkhash Lake, Kolsay lakes, Shardara, and Sorbulak reservoirs in which algal species list was compiled by us earlier. Statistical instruments will implemented for comparative floristic analysis in purpose to reveal major environmental factors that influenced algal floras forming process in arid region of Eurasia.
Description of study site
Lake Alakol and the adjacent part of the basin of its catchment area is a protected natural area. The lake is a drainage lake, belongs to the ecoregion 624 (Balkash– Alakul) according to the FEOW classification12 and once represented a single water system with Lake Balkhash. It is located at an altitude of 348 m above sea level on the Balkhash– Alakol lowland, which lies on the border of the Almaty and East Kazakhstan regions, in the eastern part of the Balkhash–Alakol depression (Figure 1). The Alakol Lake, as new included site for floristic comparison, was studied in 2015–2017. Figure 1 represent sampling points on the lake in three different areas– Kamyskala, Akshi, and Koktuma. The area of the lake is about 2,696 square kilometers, the water volume 58.56 cubic km, length 104 km, and width about 52 km. Average depth is about 22 m with 54 m maximal. The close placed lakes Sasykkol, Uyaly, Zhalanashkol and others, smaller, together with the Alakol Lake are forms the Alakol Lake system. The climate of the coast is sharply continental with a complex wind regime, ice about two month, and water temperature in range 7–25°C. The waters of the lake are brackish, chloride– sodium and chloride– sulfate– sodium, depending on the lake area, averaging about 5.9– 7.8 g l– 1.1 In the waters of Lake Alakol, the high content of fluorine and bromine.13
Algal communities were studied in altogether 34 lakes, Karasu River mouth near Lake Tuntugur, Jailma Well near Lake Kulykol, and Jarsor Brook near Lake Jarsor (most of them protected,11 which are located on the northern Kazakhstan arid area.3–6 Into analysis were included the Zerenda protected lake,7 the Alakol protected lake,2 and algal floras from large water bodies in middle and southern Kazakhstan: Balkhash Lake, Kolsay lakes, Shardara, and Sorbulak reservoirs.14–23 The species lists and environmental data for present study comes from mentioned references. GRAPHS and Statistica 12.0 programs were used for comparative floristic and dendrograms and dendrites construction.24
Environmental and biological variables for the studied lakes in Kazakhstan are presented in Table 1. Can be seen that environmental variables of Kazakhstan’s protected areas are varied in large range. Their salinity (correlated with water conductivity) varies from 0.19 tо 39.9 g NaCl per liter with salinity class from I to IV increasing during the summer dry period. The acidity varies from slightly acid to alkaline, whereas the concentration of nitrates attests to a sufficient trophic base for algal development. At the same time, the saprobity indices varied from 0.76 in Kolsay Sary–Bulak Lake to 2.7 in Aksuat Lake. Table 2 show distribution of algal diversity over taxonomic Divisions for studied lakes. As in Table 1 of environmental variables, the algal species richness is varied in wide range from minimal one species to 203 that is maximal in the Alakol Lake. Richest in the algal floras of the lakes is Diatoms, followed green algae. Total species list that was compiled for 42 studied lakes are represented by 554 taxa of species and infraspecies level. It is very interesting that most of species (about 98%) was represented by one variation only, usually typical. In Table 2 and the total list of compared floras can be seen that species composition for each flora is very individual, nevertheless wide range of environmental variables. High floras individuality can be result of protection regime in the lakes that help to survive natural algal diversity.
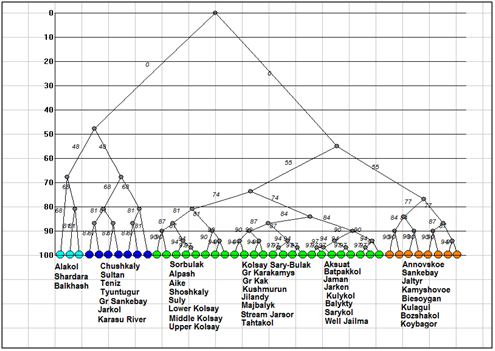
Figure 2Dendrogram of similarity for the Kazakhstan lakes algal communities. Clusters marked by different color.
We calculated algal species lists similarity for studied lakes with Sǿrensen–Chekanovsky indices and on the base of Euclidian distance constructed the tree of similarity (Figure 2) for total species richness in program GRAPHS. So, dendrogram show three different clusters on the 50% level of similarity. Cluster 1 included three largest lakes floras: the Alakol, Shardara, and Balkhash. Second cluster included richest floras of unpolluted lakes, and the third cluster unites the flora of all other lakes (Table 2).
Dendrite of inclusion– crossing for total species richness (Figure 3) demonstrated four floristic cores of algal diversity in the protected lakes of Kazakhstan. The first core with Chushkaly Lake is largest and unites richest floras of largest lakes, including of the Alakol Lake. Cores 2 represent the group of Kolsay high mountain lakes. Shoshkaly core unites the poorest flora. All other diverse floras are combined with Bozshakol Lake core. Special interest can represent the analysis of floristic similarity for the different taxonomic Division. Therefore, Figure 4 & Table 2 represents similarity of diatom floras of studied lakes. Three clusters are divided the lakes floras on the 50% of similarity. The first cluster (blue) included seven richest floras: the Alakol, Chushkaly, Tuntugur, Teniz, Gr. Sankebay, Jarkol, and the Karasu River mouth in which species number varied between 141 and 29. Second cluster (green) represent most part of studied floras, which have similarity of species list more than 73% and poorest diatom floras. Third cluster included twelve floras with similarity of 64% such as Shardara, Balkhash, Kolsay lakes and other in which have middle diatom species contain between 11 and 27. So, diatom list of similarity is repeating the tendency which has been revealed for total floras and demonstrated relatedness of similarity percent with species richness in algal floras. Dendrite for Bacillariophyta is divided algal floras for three different cores with center in flora of Koybagor (orange), Gr. Sankebay (blue), and Kolsay group of lakes. Interesting that the Alakol diatom flora is very individual and have low similarity nevertheless included to the core of Gr. Sankebay group of lakes (Figure 5). Cores of diatom floras are similar to total species richness analysis. Comparison of the Chlorophyta floras show (Figure 6) (Table 2) two major clusters on the similarity level of 50%, the first of them can be divided into two sub clusters on the similarity about 56%. So, first sub cluster (blue) included only four floras of the lakes Alakol, Balkhash, Shardara, and Karasu River mouth. Second sub cluster (light blue) combine twelve lakes with similarity of its green algal floras on the level about 72%. All 16 floras of both sub clusters are most species rich by green algae. Cluster 2 included all other lakes floras in which green algae are low presented. Figure 7 represent the calculation for an inclusion– crossing dendrite of Chlorophyta floras of studied lakes. Here can be seen that Akalol Lake flora (blue) have no similarity with most of compared floras because have small no of the Chlorophyta species. So, only three cores can be defined in the dendrite: core of Bozshakol with low green species richness, core of Alpash with minimal species number but similar to this group floras, and core of Chushkaly lake that included all other floras species. Altogether, the similarity of green algae calculation demonstrated in first low correlation with species richness and high individuality of green algal floras of studied lakes. Only one environmental variable can be mentioned as related to green algae floras similarity– the neutral or low alkaline water pH.
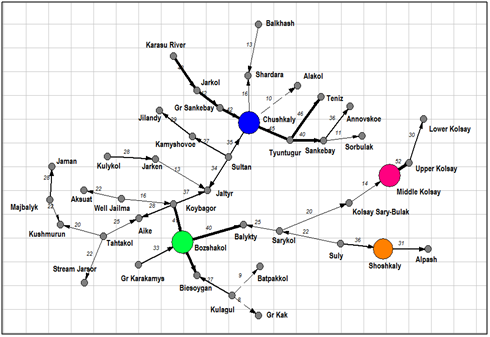
Figure 3An inclusion– crossing dendrite for floras of the Kazakhstan lakes algal communities. Floristic cores marked by different color, arrows marked inclusion direction.
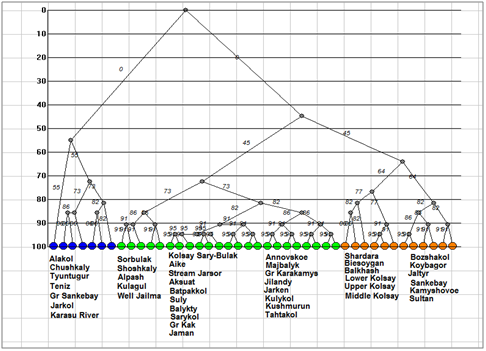
Figure 4Dendrogram of similarity for Bacillariophyta floras of the Kazakhstan lakes. Clusters marked by different color.
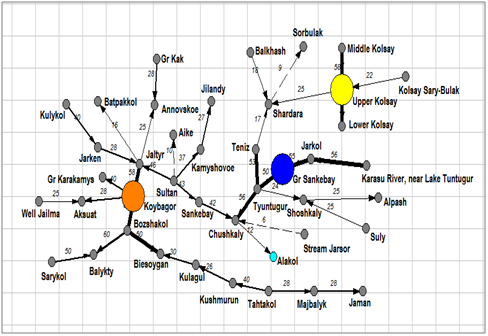
Figure 5An inclusion– crossing dendrite for floras of the Kazakhstan lakes algal communities. Floristic cores marked by different color, arrows marked inclusion direction.
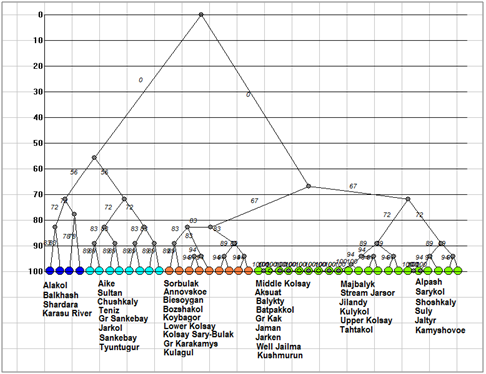
Figure 6Dendrogram of similarity for Chlorophyta floras of the Kazakhstan lakes. Clusters marked by different color.
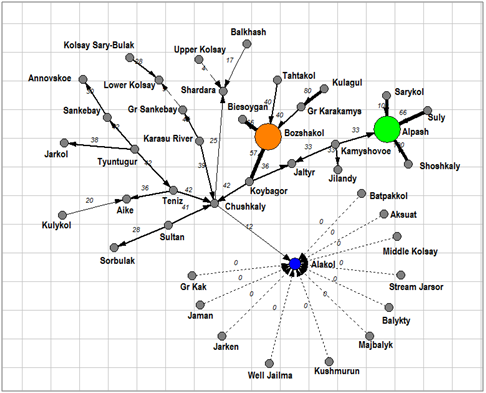
Figure 7An inclusion– crossing dendrite for Chlorophyta floras of the Kazakhstan lakes. Floristic cores marked by different color, arrows marked inclusion direction.
A special interest represent of cyanobacteria floras similarity analysis. Tree of similarity on the Figure 8 (Table 2) help us to revealed three different clusters on the similarity level about 60%. Cluster 1 included cyanobacteria from the lakes Alakol, Shardara, Karasu River mouth, Teniz, and Tyuntugur (blue). Cluster 1 floras are formed in wide range of salinity lakes (0.67– 78.6 g L– 1) and pH (6.8– 8.54) but low organic polluted and waters. Next seven cyanobacteria floras are unified into cluster 2 with similarity level about 64%. There are floras of the Balkhash, Sultan, Chushkaly, Aike, Gr. Sankebay, Jarkol, and Koybagor lakes in which also has different salinity of classes I– IV and water pH but slightly more organically polluted than lakes of cluster 1. So, we cannot see clear differences between clusters of tree. Similarity dendrite for cyanobacteria floras (Figure 9) is divided all species richness into two cores. Core 1 is formed near the lake Koybagor with Annovskoye, Sankebay and Well Jailma. These floras are characterized by middle species richness of cyanobacteria but total species richness is rather rich. We do not find some specific parameters for the lakes of this core. Core two combine all other cyanobacteria floras but the environment of this core lakes cannot be defined because in one set of floras the cyanobacterial diversity is low and in other is absent.
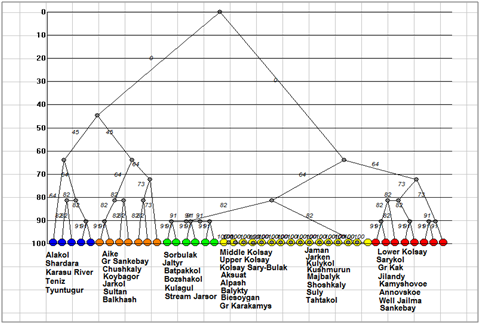
Figure 8Dendrogram of similarity for Cyanobacteria floras of the Kazakhstan lakes. Clusters marked by different color.
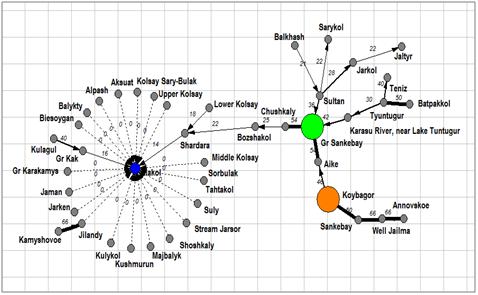
Figure 9An inclusion– crossing dendrite for Cyanobacteria floras of the Kazakhstan lakes. Floristic cores marked by different color, arrows marked inclusion direction.
Earlier we found strong correlation between species richness and water salinity class for the Northern Kazakhstan lakes.3– 6 Comparative floristic tree of the Kostanay district lakes floras has been divided for three clusters and confirms influence of salinity to species richness. More of them, when we included into calculation the lakes of wide salinity spectrum from Israel, we received the same distribution with strong relatedness of species richness and salinity.5 Therefore, we can assume that our calculation for all known Kazakhstan lakes floras can help us to classify studied lakes as salinity classes for each cluster in dendrogram Figure 2. Table 1 show that for total species richness this is not so, because the chlorides concentration in the Alakol Lake is smallest than in the lakes from same cluster Balkhash and Shardara.25 This correlation is better works for diatoms tree (Table 1,2) (Figure 4) but not for Cyanobacteria (Figure 8). The Chlorophyta species richness is highest in the freshwater lakes. Therefore, it is better indicators of salinity (Figure 6) but some influence of the lake size can be seen here also. It let us to assume that total species richness is increase with the lake size increasing but diatom and chlorophyte species richness are related to chlorides concentration more than lake volume. In respect of cyanobacteria diversity can be assumed that its species richness is increase in more freshwater lakes.
Lake |
Conductivit, mSm cm– 1 |
NaCl g L–1 |
Class of salinity |
pH |
N– NO3, mg L–1 |
No. of algal species |
Saprobity index S |
Total tree cluster |
Total dendrite core |
Aike |
11.73 |
6.79 |
III |
7.01 |
3.4 |
19 |
1.98 |
3 |
3 |
Aksuat |
4.05 |
2.32 |
IV |
6.36 |
1.2 |
1 |
2.7 |
3 |
3 |
Alpash |
8.72 |
4.92 |
IV |
7.05 |
1 |
13 |
1.74 |
3 |
2 |
Annovskoe |
0.75 |
0.44 |
IV |
6.99 |
1.6 |
22 |
1.76 |
4 |
1 |
Balykty |
4.33 |
2.43 |
IV |
7.84 |
1.1 |
26 |
2.21 |
3 |
3 |
Batpakkol |
0.37 |
0.21 |
IV |
7.29 |
2.3 |
2 |
2 |
3 |
3 |
Biesoygan |
0.85 |
0.52 |
IV |
6.76 |
2.1 |
7 |
2.55 |
4 |
3 |
Bozshakol |
0.77 |
0.46 |
IV |
6.9 |
5.1 |
63 |
2.04 |
4 |
3 |
Chushkaly |
16.4 |
9.35 |
III |
6.94 |
1.7 |
18 |
1.97 |
2 |
1 |
Gr. Kak |
57.3 |
32.7 |
II |
6.36 |
2.2 |
6 |
2.11 |
3 |
3 |
Gr. Karakamys |
1.85 |
1.08 |
IV |
6.63 |
1 |
28 |
1.73 |
3 |
3 |
Gr. Sankebay |
14.29 |
8.17 |
III |
7.4 |
1.6 |
3 |
2.49 |
2 |
1 |
Jaltyr |
11.47 |
6.52 |
III |
6.77 |
1 |
6 |
1.86 |
4 |
3 |
Jaman |
0.39 |
0.23 |
IV |
7.04 |
1.4 |
57 |
1.94 |
3 |
3 |
Jarken |
0.86 |
0.49 |
IV |
6.74 |
0.9 |
22 |
2 |
3 |
3 |
Jarkol |
5.56 |
3.26 |
IV |
7.32 |
3.2 |
8 |
1.83 |
2 |
1 |
Jilandy |
1.48 |
0.85 |
IV |
7.15 |
1.2 |
4 |
2.05 |
3 |
1 |
Kamyshovoe |
0.4 |
0.24 |
IV |
6.72 |
1.55 |
79 |
1.88 |
4 |
1 |
Karasu River |
0.4 |
0.24 |
IV |
6.86 |
1.4 |
2 |
1.47 |
2 |
1 |
Koybagor |
1.13 |
0.68 |
IV |
7.09 |
1.65 |
112 |
2 |
4 |
3 |
Kulagul |
1.02 |
0.68 |
IV |
7.53 |
0.3 |
18 |
2.41 |
4 |
3 |
Kulykol |
10.65 |
6.19 |
III |
6.96 |
1.6 |
20 |
2.08 |
3 |
3 |
Well Jailma |
1.01 |
0.58 |
IV |
7.28 |
1 |
3 |
2.35 |
3 |
3 |
Kushmurun |
30.1 |
18 |
III |
6.77 |
3.5 |
8 |
2.08 |
3 |
3 |
Majbalyk |
2.59 |
1.45 |
IV |
7.16 |
1.8 |
4 |
2.04 |
3 |
3 |
Sankebay |
14.68 |
8.8 |
III |
8.15 |
5.4 |
5 |
1.93 |
4 |
1 |
Sarykol |
1.16 |
0.68 |
IV |
6.98 |
1.2 |
37 |
1.93 |
3 |
2 |
Shoshkaly |
6.35 |
3.71 |
IV |
6.74 |
1.2 |
31 |
2.2 |
3 |
2 |
Stream Jarsor |
0.71 |
0.41 |
IV |
6.82 |
1 |
19 |
1.88 |
3 |
3 |
Sultan |
3.04 |
1.73 |
IV |
6.73 |
0.9 |
5 |
2.15 |
2 |
1 |
Suly |
0.33 |
0.19 |
IV |
7.13 |
1.4 |
7 |
1.84 |
3 |
2 |
Tahtakul |
1.65 |
0.97 |
IV |
6.57 |
1.45 |
7 |
2.25 |
3 |
3 |
Teniz |
4.76 |
2.71 |
IV |
6.68 |
1.25 |
61 |
1.92 |
2 |
1 |
Tyuntugur |
1.1 |
0.67 |
IV |
7.1 |
1.75 |
58 |
2 |
2 |
1 |
Alakol |
nd |
1.1 |
IV |
7.5 |
nd |
203 |
nd |
1 |
1 |
Balkhash |
nd |
473.3 |
I |
8.63 |
0.24 |
92 |
1.85 |
1 |
1 |
Shardara |
nd |
78.6 |
I |
8.54 |
1.36 |
78 |
1.89 |
1 |
1 |
Sorbulak |
nd |
313.7 |
I |
9 |
0.15 |
18 |
2.01 |
3 |
1 |
Kolsay Sary– Bulak |
nd |
0 |
IV |
9.6 |
nd |
6 |
0.76 |
3 |
2 |
Kolsay upper |
nd |
0.12 |
IV |
8.27 |
nd |
10 |
0.99 |
3 |
2 |
Kolsay middle |
nd |
0.08 |
IV |
8.62 |
nd |
8 |
1.12 |
3 |
2 |
Lower Kolsay |
nd |
0.16 |
IV |
8.5 |
nd |
15 |
1.51 |
3 |
2 |
Table 1 Biological and environmental variables average data for the lakes in Kazakhstan with the clusters number in total dendrogram and core number in total dendrite
Abbreviation nd – no data
Lake |
Cyanobacteria |
Bacillariophyta |
Cryptophyta |
Euglenozoa |
Miozoa |
Chrysophyta |
Chlorophyta |
Charophyta |
Total number of species |
Bacillariophyta cluster |
Chlorophyta cluster |
Cyanobacteria cluster |
Aike |
7 |
1 |
1 |
– |
– |
– |
9 |
– |
18 |
2 |
1 |
2 |
Aksuat |
– |
1 |
– |
– |
– |
– |
– |
– |
1 |
2 |
2 |
3 |
Alpash |
– |
10 |
– |
1 |
– |
– |
1 |
– |
12 |
2 |
2 |
3 |
Annovskoe |
2 |
5 |
– |
2 |
– |
– |
7 |
4 |
20 |
2 |
2 |
3 |
Balykty |
– |
4 |
– |
– |
– |
– |
– |
– |
4 |
2 |
2 |
3 |
Batpakkol |
1 |
1 |
– |
– |
– |
– |
– |
– |
2 |
2 |
2 |
3 |
Biesoygan |
– |
21 |
– |
– |
– |
– |
3 |
1 |
25 |
3 |
2 |
3 |
Bozshakol |
1 |
7 |
– |
1 |
– |
– |
3 |
– |
12 |
3 |
2 |
3 |
Chushkaly |
7 |
29 |
– |
1 |
– |
– |
15 |
1 |
53 |
1 |
1 |
2 |
Gr Kak |
4 |
2 |
– |
– |
– |
– |
– |
– |
6 |
2 |
2 |
3 |
Gr Karakamys |
– |
4 |
– |
– |
– |
– |
2 |
– |
6 |
2 |
2 |
3 |
Gr Sankebay |
4 |
29 |
– |
3 |
– |
– |
17 |
2 |
55 |
1 |
1 |
2 |
Jaltyr |
2 |
11 |
1 |
1 |
– |
2 |
7 |
2 |
26 |
3 |
2 |
3 |
Jaman |
– |
2 |
– |
– |
– |
– |
– |
– |
2 |
2 |
2 |
3 |
Jarken |
– |
3 |
– |
1 |
– |
– |
– |
– |
4 |
2 |
2 |
3 |
Jarkol |
7 |
40 |
1 |
2 |
– |
– |
16 |
10 |
76 |
1 |
2 |
2 |
Jilandy |
1 |
4 |
– |
– |
– |
– |
1 |
– |
6 |
2 |
2 |
3 |
Kamyshovoe |
2 |
18 |
– |
– |
– |
2 |
5 |
1 |
28 |
3 |
2 |
3 |
Karasu River |
10 |
45 |
1 |
6 |
1 |
– |
36 |
4 |
103 |
1 |
1 |
1 |
Koybagor |
6 |
6 |
1 |
– |
– |
– |
4 |
– |
17 |
3 |
2 |
2 |
Kulagul |
1 |
12 |
1 |
– |
– |
1 |
3 |
1 |
19 |
2 |
2 |
3 |
Kulykol |
– |
2 |
– |
– |
– |
– |
1 |
– |
3 |
2 |
2 |
3 |
Well Jailma |
1 |
7 |
– |
– |
– |
– |
– |
– |
8 |
2 |
2 |
3 |
Kushmurun |
– |
3 |
– |
1 |
– |
– |
– |
– |
4 |
2 |
2 |
3 |
Majbalyk |
– |
5 |
– |
– |
– |
– |
– |
– |
5 |
2 |
2 |
3 |
Sankebay |
2 |
13 |
1 |
2 |
– |
– |
13 |
4 |
35 |
3 |
1 |
3 |
Sarykol |
2 |
1 |
– |
– |
– |
– |
1 |
1 |
5 |
2 |
2 |
3 |
Shoshkaly |
– |
6 |
– |
– |
– |
– |
1 |
– |
7 |
2 |
2 |
3 |
Stream Jarsor |
1 |
1 |
1 |
– |
– |
– |
– |
– |
3 |
2 |
2 |
3 |
Sultan |
7 |
19 |
– |
1 |
– |
1 |
9 |
2 |
39 |
3 |
1 |
2 |
Suly |
– |
2 |
– |
– |
– |
– |
2 |
– |
4 |
2 |
2 |
3 |
Tahtakol |
– |
2 |
1 |
1 |
– |
– |
2 |
– |
6 |
2 |
2 |
3 |
Teniz |
7 |
29 |
– |
– |
– |
1 |
13 |
4 |
54 |
1 |
1 |
1 |
Tyuntugur |
3 |
35 |
– |
3 |
– |
– |
15 |
2 |
58 |
1 |
1 |
1 |
Alakol |
20 |
141 |
– |
6 |
– |
– |
16 |
20 |
203 |
1 |
1 |
1 |
Balkhash |
21 |
27 |
– |
4 |
3 |
2 |
29 |
4 |
90 |
3 |
1 |
1 |
Shardara |
8 |
16 |
– |
– |
8 |
3 |
41 |
2 |
78 |
3 |
1 |
2 |
Sorbulak |
2 |
6 |
– |
– |
3 |
– |
5 |
2 |
18 |
2 |
2 |
3 |
Kolsay Sary– Bulak |
– |
1 |
– |
– |
– |
– |
3 |
1 |
5 |
2 |
2 |
3 |
Upper Kolsay |
– |
8 |
– |
– |
– |
– |
2 |
– |
10 |
3 |
2 |
3 |
Middle Kolsay |
– |
9 |
– |
– |
– |
– |
– |
– |
9 |
3 |
2 |
3 |
Lower Kolsay |
3 |
7 |
– |
2 |
– |
– |
4 |
– |
16 |
3 |
2 |
3 |
Table 2 Distribution of species richness in taxonomic division over studied lakes of Kazakhstan
Total species richness comparative floristic analysis demonstrated importance of the water body size, which correlated with species number in the lake floras. It can better see in the diatom floras comparison cluster tree of which is related to the lake salinity class also. Cyanobacteria and Chlorophyta species richness are highest in the freshwater lakes as can be seen in its similarity tree. Therefore, it is better indicators of salinity but some influence of the lake size can be seen here also. So, total species richness is increase with the lake size increasing but diatom and chlorophyte species richness are related to chlorides concentration more than lake size. The Cyanobacteria tree demonstrated increasing of species richness in freshwater lakes. From other hand, we found that inclusion– crossing dendrites for total flora of studied lakes as well as for different taxonomic Division can be used for the algal floristic forming process in Kazakhstan. Total algal floras analysis with revealing of floristic cores show two major cores in which is concentrated of species diversity. One of them is related to freshwater and second to special high mountain environment. The same distribution is demonstrated by diatom and chlorophyte floras dendrites. However, cyanobacteria dendrite revealed correlation of core members with the salinity Class of the lake. Therefore, we can to conclude that salinity and the size of the lake play the major role for species richness in the Kazakhstan lakes flora forming process. More of them, comparative floristic analysis revealed the tendencies in forming of the Kazakhstan diversity that is influenced by arid climatic factors such as high evaporation and salinity of the lakes increasing.
This work was partly supported by Committee of Science, Ministry of Education and Science, Republic of Kazakhstan as well as by the Israeli Ministry of Aliyah and Integration.
The author declares there is no conflict of interest.

©2018 Jiyenbekov, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.