MOJ
eISSN: 2573-2919


Research Article Volume 2 Issue 4
Research Center for Environmental Changes, Academia Sinica, Taiwan
Correspondence: Amzad Hussain Laskar, Research Center for Environmental Changes, Academia Sinica, Taipei, Taiwan, Tel 886 -265-398-85
Received: May 09, 2017 | Published: June 21, 2017
Citation: Laskar AH. Conventional and clumped isotopes in ecological research. MOJ Eco Environ Sci. 2017;2(4):148-156. DOI: 10.15406/mojes.2017.02.00028
Stable isotope ratios at natural abundance levels are increasingly used in ecological studies. Common uses of stable isotopes in ecology include their use as biological tracers for source identifications, tracing nutrient and contaminant flows in food webs, identifying the geographic origin of migratory animals and birds, studying cross-continental spread of infectious diseases, diet reconstruction of extinct animals, food authentication, study of photosynthesis by different types of plants, estimating CO2 emissions from different sources to the atmosphere and quantifying relative inputs in a system. Here we review some of the applications of stable isotopes in ecological and pale ecological studies. The review is restricted to the study of natural variations of the isotopes. Recently developed advanced stable isotope tools such as clumped and triple oxygen isotopes are also introduced in ecological studies. Potential of clumped isotopes to understand the thermal physiology of dinosaurs is discussed in this review.
Stable isotopic composition of carbon, nitrogen, oxygen and hydrogen are increasingly used in ecological and environmental studies. The advantage of stable isotope data in biogeochemical research is that they can contribute to both source-sink (tracer) and process information. They provide ecological information across a range of spatio-temporal scales, i.e. from cell to ecosystems, and across a time scale of seconds to millennia [1]. With modern mass spectrometers isotopic compositions of natural materials can be measured with extremely high precision. As elements cycle through the biosphere, isotopic compositions change in predictable ways. These changes have been exploited by geochemists to understand the global elemental cycles. This review is to give a brief idea about the basic applications of stable isotopes in ecosystem studies. It is started with a brief description of the stable isotope measurement techniques along with the natural variations of the isotopic compositions of common elements. It is focussed mainly on C, O, N and H isotopic analysis. Also a potential application of a recently developed advanced technique viz. clumped isotope thermometry in ecosystem and pale ecology research has been discussed.
Isotopes of an element have the same number of protons but differ in their number of neutrons, resulting in different masses. There is variation in the relative abundances of the isotopes. The lighter isotopes generally form weaker bonds than the heavier ones and tend to react faster causing a slight difference in their relative abundances in different phases and in the products of different chemical and physical processes. This change in the isotopic abundance is called fractionation. Different environments are often characterized by predictable isotopic signatures. Stable isotopes are measured in mass spectrometers as isotopic ratios and are expressed as isotopic deviations from standards, denoted by delta (δ) values as parts per thousand or per mil denoted by ‰. The δ values are calculated as follows:
δX = [(Rsample/Rstandard) – 1]× 1000
Where X is the isotope (such as 13C and15N), and R is the corresponding isotope ratio (13C/12C or 15N/14N). The quotient of the ratios in the sample relative to the standard is the δ value. Each element has a primary international standard with a δ value of 0‰. Deviations towards the positive and negative imply enrichments and depletions in the heavy isotope respectively, with respect to the standard. Measurements are carried out in gas phase; samples are converted into gases and purified before introducing into the mass spectrometer. The precision of measurements varies from gas to gas. With modern mass spectrometers, precisions better than 0.1 ‰ is easily achievable for δ13C and δ18O. Anomaly in 17O in CO2 is another recently introduced tracer and is mostly used to identify the sources of CO2 [2-5]. It is the deviation of d-values of a sample from the mass-dependent fractionation line expressed by D17O = ln(1+d17O)-λ´ln(1+d18O). The factor λ vary between 0.500 and 0.529 depending on different fractionation process [6]. With recently developed analytical techniques, D17O can be measured with a precision better than 0.01 ‰. Similar to 17O, clumped isotopes in CO2 (excess of mass 47, mainly 13C18O16O over a random distribution of isotopes, denoted by Δ47) is another powerful tracer and can be used to identify the sources of CO2 [7]. Δ47 is defined by
where R13 and R18 (ratios 13C/12C and 18O/16O) are obtained by measuring the traditional masses 44, 45 and 46 in the same CO2 sample and R17 is calculated assuming a mass dependent relation with R18 (for details, see [7,8-10]. D47 can be measured with a precision better than 0.01 ‰.
Stable carbon isotopes
Carbon has two naturally occur ring stable isotopes: 12C and 13C with abundances of 98.93% and 1.07% respectively. The carbon isotopic composition (δ13C) is expressed with respect to international standard Vienna Pee Dee Belemnite (VPDB) derived from a Cretaceous cephalopod (PDB). δ13C in natural samples can have variations of several tens of per mils with very negative values in biogenic methane and other reduced carbon compounds to marine carbonates with around zero values. One of the seminal discoveries in stable isotope ecology was differential discrimination during photosynthesis by different kind of plants [11]. About 85 % of plants follow Calvin cycle or C3 cycle (most of the big plants and cool season temperate plants) with δ13C in the range of -34 to -20 [12,13]. Less than 0.5 % plants follow Hatch-Slack cycle or C4 cycle (tropical warm grasses, sugarcane, sorghum etc.) with δ13C in the range of -9 to -19 ‰ [14]. A third kind of photosynthetic pathway called Crassulacean Acid Metabolism or CAM (grow in dry environments such as cacti and pineapples) with intermediate δ13C between C3 and C4 plants consists of ~10% of the plants [15]. The difference in carbon isotopic composition is caused by the fact that the ribulose-1,5-bisphosphate carboxylase (Rubisco) catalyst, involved in the C3 pathway strongly discriminates against 13CO2 compared to phosphoenolpyruvate (PEP) carboxylase, the C4 catalyst. Carbon isotopes cannot be used to distinguish CAM from C4 plants because of their similarity. However, evidences suggest that the CAM plants are considerably enriched in deuterium relative to source water. Therefore, hydrogen isotopic composition (δD) is an important tracer to distinguish CAM plants from C3 and C4 plants [16-20] which traditionally was done by anatomical methods, specifically succulence (CAM) vs. Kranzanatomy (C4). More details about the applications of hydrogen isotopic composition are given in a later section.
Soil organic matter reflects the type of vegetative cover present in a given soil [21-25]. A simplified diagram of the distribution of δ13C in ecosystems is shown in Figure 1. δ13C is also widely used for predicting the origin of diets through time and across space [26-30]. This is because animals are similar in isotopic compositions to their diets for carbon but heavier by 3-5 ‰ from dietary nitrogen. Thoughstable isotope ratios do not reproduce exact menu, but broadly help to constrain food categories such as, meat versus plants, terrestrial versus aquatic protein sources and C4grasses/cereals versus C3 fruits/vegetables [31-33]. Figure 2 gives a brief idea of the isotopic variation in the food web of terrestrial and aquatic plants. Study of human diets in ancient settings is a key to understand the Cultural revolution and human modification with time in ancient ecosystems. Stable isotopes in the fossil bone collagen, teeth enamel, bipartite and eggshell carbonate are useful to study the ancient human diets. Understanding the diet and nutrition is carried out by analysing stable isotope along with other physiological factors which influence the stable isotope ratios in the body. This helps our understanding of health and nutrition over a broad range of contexts [34-38].
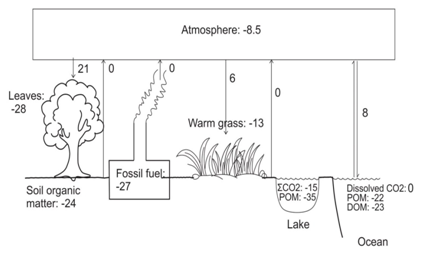
Figure 1: Carbon isotopic distribution in ecosystems, the numbers in various reservoirs is their approximate δ13C values in ‰.
CO2 fluxes are shown by single arrows; the numbers next to the arrows are the approximate fractionations during the transfer of CO2. Double arrow indicates equilibrium fractionation. POM: Particulate Organic Matter; DOM: Dissolved Organic Matter [2,7,12,13,22,24,34-38].
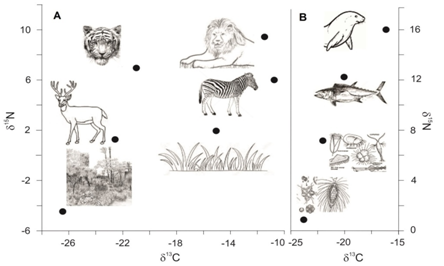
Figure 2: A simplified diagram showing trophic enrichment in carbon and nitrogen isotopic compositions from primary producers to herbivores to predators for (A) Terrestrial ecosystem and (B) Marine ecosystem. For terrestrial ecosystem, food chain starting with both C3 and C4 plants as primary producers are shown [57].
Stable nitrogen isotope
Nitrogen is mainly present as N2 gas in the atmosphere with δ15N of 0‰. Stable isotope studies of nitrogen are helpful in identifying the sources and fates of N.Nitrogen isotopes fractionate during primary uptake. Based on their source of nitrogen, plants may be divided into two types: those can utilize N2 directly, and those utilize only “fixed” nitrogen as ammoniaand nitrate. The former include the legumes (e.g., beans, peas) and marine cyanobacteria.Very little nitrogen isotopic fractionation takes place during direct fixation of N2 from atmosphere and hence δ15N values for such plants are close to zero [e.g., 39,40]. The legumes, which are exclusively C3 plants, utilize both N2 and fixed nitrogen (though symbiotic bacteria) have average δ15Nvalue of +1‰ [41,42]. Marine plants have δ15N valueof 5 – 10 ‰ and marine cyanobacteria have δ15N of -1±3 ‰ [43-45]. Through biological N2 fixation, assimilation atmospheric N2 into NH3 by some specific microorganisms is a key process for the atmospheric N2 to enter into terrestrial ecosystem [46]. There is a wide range of isotopic values for nitrogen in ammonia and nitrate [47]. Therefore, Non-nitrogen-fixing plants have a more varying range of δ15N values depending on their source of nitrogen. However, very little is known about the N2 isotopic fractionation during assimilation of organic form of nitrogen. Plants can be slightly enriched or depleted in 15N compared to the NO3 source [48] probably due to release of nitrogen fractionated compounds [49]. Successive oxidations steps in nitrification are associated with varying amounts of kinetic fractionations. Most of the organic nitrogen in soils is slowly converted into ammonium, the rate-determining step in the nitrification process and the resulting nitrate is quite similar in terms of δ15N to the organic starting materials. However, when large amounts of ammonium are available, the oxidation steps to nitrite and nitrate are rate limiting, and the nitrate formed may be very depleted in 15N. Nitrogen isotopic fractionation may be induced by high external NO concentrations, osmotic stress, or drought, although the mechanisms for this fractionation are still not known very well [50,51]. On average modern nonleguminous plants have average δ15N value a few per mil higher than the atmospheric N2[1]. δ15N values of different plant parts can differ by 2-3 ‰ but can be as high as 7 ‰ in desert plants [52]. Isotope label experiments such as supplying 15N enriched nitrate compound and following its fate can be a way to differentiate between external and internal plant sources and to quantify the importance of the internal cycling of nitrogen. Dual-isotope (say 13C and 15N) is also a powerful tool to demonstrate the relationship between internal N stores and recently fixed photosynthate [53].
Denitrification back to molecular nitrogen gas involves large15N depletion [54]. All the fractionations in the pedospheric part of the nitrogen cycle are heavily dependent on nitrogen concentrations. Synthetic fertilisers, since being produced from air via the Haber process, are close to 0‰ [55]. Volatilisation of ammonium compounds to ammonia from manure, however, involves a fractionation of up to -40‰, such that the residue may be very enriched in15N. Figure 2 shows changes in carbon and nitrogen isotopic compositions in terrestrial and marine food chain.A comprehensive review of nitrogen isotope fractionations in plants and soils can be found in Högberg [56].
Stable oxygen and hydrogen isotopes
Oxygen isotopic composition (δ18O) is widely used for partitioning net CO2 terrestrial fluxes between soil respiration and that exchange with plant leaves, the exchange rate is enhanced by catalytic action of carbonic anhydrase in plants and soils [57-64]. This is because the δ18O of CO2 fluxes originated from soil respiration are different from that exchanged with leaf water. δ18O in soil water reflect the δ18O value of meteoric water but the leaf water is relatively enriched due to transpiration. As the δ18O values from these processes and interactions are different, therefore, this tracer is widely used for constraining the gross production of CO2 [58,61,64-66]. δ18O in ecosystems are also used for identifying the source of water. It can be used for phosphate sources in aquatic ecosystems by measuring the δ18O values in dissolved inorganic phosphates [67]. δ18O in phosphates, bioaptites, tooth enamels and eggshell carbonates are widely used to reconstruct the drinking water of extinct species such as dinosaurs [68,69].
Stable hydrogen isotopic composition (δD)is very useful to study the origin of migratory animals specifically birds as there is geographical pattern of δD in the precipitation. It is observed that the δD values in bird’s feathers are correlated with the δD values of the precipitation and hence this isotope proxy is widely used for assigning origin of migratory birds [e.g., 70-73]. Though δDhasso far been restricted mainly to studies of migratory origin and pale climate reconstruction based on systematic relationships between organism tissue and local environmental water, it can be used to study modern food webs as demonstrated recently. The large δD differences between aquatic and terrestrial ecosystem end members can be utilized for the quantification of energy inputs and nutrient fluxes between these two sources [74]. Particularly δD can be used for determining allochthonous vs. autochthonous nutrient sources in freshwater systems. Some studies also suggested that δ Dhas relation with trophic position and this marker may be used as a trophic indicator, in addition to the more commonly used δ15N. Coupled measurements of δ2H and δ18O values are increasing as a result of advancement of precise analytical techniques to measure both simultaneously, which may provide additional ecological information over single element measurements.
Triple oxygen and clumped isotopes
CO2 in the atmosphere has many sources and sinks with diverse fluxes. Because of this diversity and variability, it is always not possible to constrain the sources and sinks using conventional isotope data. For example, it is not possible to distinguish CO2 emitted from fossil fuel burning and respired CO2 from C3 plants or soil under C3 vegetation cover as δ13C value of the two sources are similar. Similarly, rapid exchange of oxygen isotopes between CO2 and different water reservoirs with diverse δ18O and processes such as vapor transpiration complicate its interpretation [75]. In such cases triple oxygen and clumped isotopes can be very useful [76,2] .Plant and soil respired CO2 has Δ17O value close to zero while CO2 from combustion sources has negative Δ17O making it ideal for distinguishing the two sources. Δ17O in stem and leaf waters in plants has also been found to be very useful to understand transpiration processes under different relative humidity condition [4]. Clumed isotope (Δ47) values of CO2 depend on the formation temperature of the CO2. When CO2 interacts with water, Δ47 value of the CO2 gets modified depending on the temperature of the water. At thermodynamic equilibrium, CO2 is expected to reflect the temperature at which exchange takes place [77,78]. Thus the fluxes that involve CO2 equilibration with water have Δ47as expected at thermodynamic equilibrium. Similar to Δ17O, Δ47 is also very useful to distinguish CO2 originated from respiration and combustion. Also Δ47 is found to be sensitive to photosynthesis making it suitable to study photosynthesis also [7]. Triple oxygen isotopic data in biogenic carbonates (eggshell carbonates, bioapatite and tooth enamels) are useful to study influence of evaporation and metabolic waterin animal body [79-81]. A strong environmental and physicological control on body water Δ17O has been observed for modern mammals and birds [81]. Therefore, this could be used for pale environmental and pale ecological reconstruction. Clumped isotopes in carbonates directly give the temperature of precipitation. Therefore, clumped isotope analysis in biologically precipitated apatite in bone, teeth and eggshell carbonates can shed light on the thermal physiology of extinct animals such as dinosaurs [82-84] which was previously carried out indirectly [82-92].
Some applications of stable isotopes in ecological and paleoecological problems
Applications of stable isotopes in ecological research are very diverse.Some specific examples of the applications of a few selected stable isotopes are given below.
Stable carbon and nitrogen isotopes in determining animal’s diet
Stable isotope analysis is very useful to trace modern and extinct animal’s diet. Stable isotope gives information of diet integrated over a longer time period and offers an alternative approach which is often nondestructive and in many cases noninvasive. Animals are similar in isotopic compositions to their diets for carbon, but on average 3 to 5 ‰ heavier than dietary nitrogen. Diet-switching experiments for carbon clearly show that the diet is the primary determinant of animal isotopic compositions [93-96]. For example, gerbils that were switched from corn diet (δ13C= -12 ‰) to wheat-based diet (δ13C= -22‰) approached the wheat carbon isotopic composition with time though the turnover times are different in different tissues [96]. The fastest change was observed in liver (half-life 6.4 days) and slowest in hair (half life 47.5 days). Thisis also true for nitrogen, with the caveat that animals are somewhat enriched in 15N compared its value in diet and the magnitude of the difference is relatively consistent among organisms [97-100]. 15N enrichments versus diet are found to be related to processes of nitrogen assimilation and excretion. More detail discussion regarding this enrichment can be found in previous works [e.g., 99,101,102].
Variation of atmospheric CO2 levels using stable isotopes in tree rings
CO2 is the main greenhouse and its rapid increase in the atmosphere in the industrial era is the central point of the concern for climate scientists. For any mitigation strategy, it needs proper understanding the sources of CO2. The relative contribution of CO2 from combustion of fossil fuels and from oxidation of forest and soil organic matter particularly due to deforestation and land-use changes has been debated [103,104]. While history of CO2 emitted from fossil fuel combustion is known very well, the timing and amount of CO2 released from the terrestrial organic matter is not known well. The terrestrial contribution of CO2 to the atmosphere can be estimated using models and 13C changes with time in the past.
Addition of CO2 from the fossil fuel combustion with average δ13C value of ~-27 ‰ [2] and oxidation of terrestrial organic matter with average δ13C value of ~-28 ‰ [22,25] has lowered the overall δ13C value of the atmospheric CO2. As the amount of fossil fuel burned at different times is well known, it is possible to calculate the amount of CO2 released to the atmosphere by terrestrial biomass in the past, particularly during the industrial period. The model needs accurate time course of atmospheric δ13C values as the CO2 continuously exchanges with oceanic and biospheric carbon. Tree rings can provide accurate δ13C values in the atmosphere in the past. A major advantage with tree rings is that they can be precisely dated with annual or even seasonal time resolution [105]. Though there is variation in the δ13C values from tree to tree, overall a decrease of δ13C by 1.8 ‰ has been recorded from the beginning of the industrial period (~1750 CE) to the year 2000 CE [106]. Analysis of atmospheric CO2 trapped in polar ice core air have supported the timing and magnitude of atmospheric δ13C changes deduced from tree ring analysis [107,108]. Variation in the δ13C values derived from tree rings during the last ~250 years is shown in Figure 3A [109]. The variation in atmospheric CO2 concentration derived from polar ice core during the same period is shown in Figure 3B. A significant decline in the δ13C values was observed during 1800s, well before the upswing of the fossil fuel use. This has been attributed to long-term and large-scale release of CO2 depleted in 13C due to clearing and burning of forests and oxidation of soil organic matter (with δ13C value of ~ -28 ‰). However, expected increase in the corresponding CO2 concentration is not very obvious probably because of the dampening of the signal in the polar region and coarser resolution of ice core data. The recent rapid decrease in δ13C correlates with the increase in fossil fuel use. An obvious conclusion is that the release of CO2 from terrestrial carbon stocks to the atmosphere was greatest during 1800-1920 AD. A steady decrease in the δ13C values between 1920 to 1960 AD was caused by both terrestrial and fossil fuel burned CO2. Model calculations showed that the contribution of CO2 to the atmosphere from the two sources is comparable until 1980 AD: ~ 140×109 tons from biosphere and 170×109 tons from fossil fuel combustion [110]. The sharp decline in δ13C and increase in CO2 concentration post 1960 was mainly due to ever increasing rate fossil fuel combustion.
This example illustrates the usefulness of isotope tracers in paleoecology and climate change research. Alternative methods of calculating the loss of biospheric carbon through analysis of land-use patternsand deforestation are not very accurate due to the lack of accurate historical land-use records. More than one independent methods of computingchanges in biospheric carbon stocks (δ13C versus historical land use records) is important considering the importance of properunderstanding of thecontrols of atmospheric CO2 levels [111].
Identifying the geographic origin of migratory birds
Stable isotopes in animal keratins including hair, claw, skin, nail, horn, baleen and feathers have been increasingly used for studying animal migration [57,72,112]. Keratin is metabolically inert and once formed, the chemical composition including its isotopic signature reflects the environmental conditions under which it was developed. If an animal molts in one location and then migrates across any of the isotope landscapes, the isotopic signature held in keratin becomes a migratory tag that can be used to predict where that animal was when the keratin grew. Since the pioneering studies by Best and Schell (1996) [113], Chamberlain et al. (1997) [114] and Hobson and Wassenaar (1997) [115] using stable isotopes to track migration by identifying systematic geographic pattern in the isotope signatures in tissues from wild animal populations, stable isotopes have been widely used to elicit spatiotemporal structure in patterns of mammalian migration in both marine [113,116,117] and terrestrial [118] systems. Stable hydrogen isotopes (δD)are useful to study patterns of migration and connectivity for birds [119] and bats [120]. δD hasalso been widely used in humanforensics applications [121,122]. The main reason behind the use of these isotopes to study migration is that, these isotopes in general have predictable compositions over a geographic region and follow some patterns with geographical and other ecologically meaningful gradients.However, it is not straightforward to assign the geographic origin of migratory animal using isotopes because of many complicacies such as assigning isotopic values to a given region by measurements or interpolation and the variation of the isotopic composition in different individuals in a given location. Therefore, multiple isotope proxies are often better in assigning the geographic origins [e.g., 73,123].
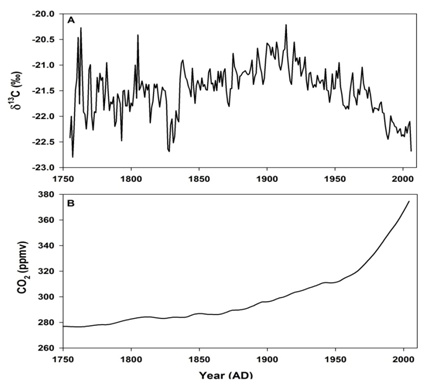
Figure 3A: Stable carbon isotopic composition (δ13C) derived from African tree ring [109].
Figure 3B: Atmospheric CO2 concentration during the industrial period (from 1750 to 2010 AD) derived from Law Dome Ice Core, Antarctica [111].
(source: World Data Service for Pale climatology, Boulder and NOAA Pale climatology Program National Centers for Environmental Information (NCEI)).
The most important step for using stable isotopes for tracking animal migration is to calibrate an assignment model to link isotopes and geography using tissues of known origin (Wunder and Norris 2008). This is done either using sample data of a region without defining any mechanisms for predicting isotope values of the region [124,125] or smoothed or interpolated patterns that generate predicted results across the full range of spatial scales for possible geographic locations [e.g., 126]. Wunder et al. [73] used feathers of known origin collected across the breeding range of migratory mountain plover to test the use of isotope tracers for assigning breedingorigins. One year data was used to generate the model and next year data to test the model performance. They analyzed δ13C, δ15N and δD in feathers of the bird and observed a systematic variation of δD in the feathers with latitude variation of 6 to 9 degree indicating that hydrogen isotopes in birds feathers is a good tracer for studying the origin of migratory birds. However, no strong systematic δ13C variation in the feathers with latitude was observed. This is due to combination of C3 and C4 vegetation used by mountain plovers. Probably local vegetation, geography and demography affects the δ13C values more than regional to continental effects as demonstrated by Graves et al. [127]. Nitrogen isotopic composition (δ15N) in plover’s feather showed a systematic variation with latitude. Inverse regression model was found to be inappropriate to assign latitudinal origin of the birds, instead they used the probability density estimated from data generated in 2001 to assign the geographic origin of the birds from the data generated in 2002. Combined isotopic data were found to better assign of the geographic origin of the bird than that predicted by any of the single or double isotope data. Caccamise et al. [71] carried out measurements of carbon, nitrogen and sulphur isotopic compositions on newly grown feathers in birds of known origin in a single year.The sample groupings were stratified across clear discrimination gradients in the three studied isotopes and they reported a 92% correct assignment rate. Hobson et al. [72] showed that δD and δ18O in feathers of mmigratory birds in Europe are useful to track their origin and movements. Wassenaarand Hobson (2000) [128] correctly classified 80% oftheir data among 11 locations that spanned discriminationgradients in carbon and hydrogen isotopes even though the feathers werecollected and pooled over a 17-year period from uncertain locations. Royle & Rubenstein [129] pooled feathers for 10 years from uncertain origins and categorised into three vast geographic regions, eachrepresented by three collection sites. Using carbon and hydrogen isotopes, 62% of the observationswere assigned to the correct region.
With availability of more spatially resolved isotopic data, the assignment of the geographic origin of birds is becoming more accurate. It is relatively straightforward to convert isotopic cluster pattern to other tissue isocapes (bone collagen, hair, muscle) by applying the appropriate discrimination factors. Similarly, our understanding of isotopic discrimination factors linking feathers to food web or precipitation baselines are likely to improve over time with new research and better techniques which would help to revise the mean tissue values associated with the isotopic clusters. Another potential means to adopt a multivariate approach is to combine stable isotope analysis with trace element profiles of feathers or hair to trace the origin of birds. Trace elements in feathers reflect that of the soil substrate where feathers were grown. With analytical advancements, fingerprinting individuals using trace elements is gaining interests and a combined study would provide better means to trace the origin of individuals.
Clumped isotopes to constrain thermal physiology of dinosaurs
The body temperatures of dinosaurs provides crucial information in tracing biological evolution of thermoregulation. Since the discovery of dinosaurs in 1842 CE, there has been discussion about the nature of their body metabolism, particularly whether they were endothermic or ectothermic. Initially dinosaurs were believed to be ectothermic similar to modern day reptiles that derive heat from the environment to maintain their body temperatures, in contrast to endothermic mammals and birds which maintain almost a stable body temperature by internal metabolic heat production. In the later part of the 20th century, evidences in support of the endothermic metabolism started emerging from observations of their behavior, pale geographic distribution, and anatomy of non-avian dinosaurs [85,130,131]. Until recent time the thermal physiology of non-avian dinosaurs, especially the endothermic/ectothermic nature of their metabolism were inferred indirectly using body mass, biophysical modeling, bone histology, and growth rate through assumptions which remain highly debated. Clumped isotope thermometry, based on the thermodynamically driven preference of 13C-18O bond in carbonate minerals of fossilized bioapatites, tooth enamels and eggshells allows a direct measurement of their body temperatures, providing a new window in understanding the thermoregulations of the extinct oviparous species. The advantage of clumped isotopes is that the isotope signature (13C-18O bond abundance) in carbonate lattice is dependent on the precipitation temperature only, unlike conventional stable isotope paleo-thermometers such as oxygen isotope thermometry which is dependent on the temperature of precipitation as well as the isotopic composition of water from which the mineral formed. It is found that the 13C-18O bond abundances in the carbonate component of tooth bioapatite and eggshell carbonate from modern specimens follow the same relationship between isotope clumping and temperature and can reproduce the body temperature with accuracy better than 2 ° C. This thermometer is being applied to constrain the thermal physiology of extinct species such as dinosaur and this is the first direct measurements of their body temperatures. The main concern is to ensure that the fossilized material were not altered, called digenetic alteration during their burial. Such alterations of 13C-18O bond ordering are possible through dissolution/re-precipitation reactions associated with intrusion of secondary carbonate solution. However, it is possible to check the preservation of eggshell carbonates subsequent to their burial. Usually Scanning electron microscope (SEM) analysis is carried out on the carbonates to check if the primary cell structure remained unchanged, an evidence of the preservation of samples. Another test is to measure the isotopic composition including clumped isotopes in the sample and the host rock. For well preserved samples, a significant difference in the isotopic values between the samples and host rock is expected. Simple plot between δ13C and δ18O is also used to check their preservation: for altered samples a significant correlation is expected as the alteration may effect both the isotopes simultaneously.
Eagle et al. [84] measured Clumped isotope values (Δ47) in Late Cretaceous titanosaurid eggshells collected from three different sites: the Nemegt Basin, Mongolia, Auca Mahuevo in Neuquén Province, Argentina and the Provence Basin in France to estimate their body temperatures. Before applying the clumped isotope thermometry to fossil eggshells of dinosaurs, they verified the clumped isotope thermometry with modern eggshells by measuring the Δ47 values in the eggshell carbonates and comparing the actual body temperatures for a series of modern oviparous animals. They observed Δ47 values in the range of 0.65 to 0.67 ‰ for well-preserved eggshells of the Late Cretaceous dinosaur species. These values give dinosaur body temperatures in the range of 30 to 35 ° C. The highest body temperature is comparable to the body temperature of modern mammal but on average ~6 o C less than modern birds implying that this tax on did not have thermoregulation comparable to modern birds, but was able to elevate its body temperature above environmental temperatures. Therefore, they concluded that probably dinosaurs cannot be categorised to any of the end-member ectothermy or endothermy but maintained a variable thermoregulation. Similar conclusion could be drawn from their previous analysis of clumped isotopes in bio minerals [82,83]. For a better constrain on the thermoregulation of this extinct species, more clumped isotope analysis on well preserved eggshells with wide spatial distribution is required. More such analysis from other groups are expected to come in future which will not only help to constrain the thermoregulation of this extinct species but also their evolution and extinction [130,131].
Stable isotopes distributions in natural systems reflect an integrated history of physical and metabolic processes within ecosystems and are very useful for a wide variety of ecosystem studies. This review provided information on how stable isotopes can be used to various aspects of ecological research. The discussion is limited to applications of some selected isotopes. Additional measurements of some more stable isotopes such as sulphur and strontium are found to be useful in some circumstances though not discussed in this review. Clearly, the combination of several independent isotopic tracers shows the greatest promise in addressing an ecosystem problem. Advancements in analytical techniques especially precise measurements of multiply-substituted isotopes has open a new horizon for addressing many more ecological and paleo-ecological problems such as thermoregulation of dinosaurs as discussed in this review. Another recently established clumped isotope proxy in methane may help to constrain its formation particularly, to distinguish biogenic methane from thermogenic origin.
The author is thankful to Dr. Aylen Green for inviting to contribute an article in the Journal MOJ Ecology and Environmental Science.

©2017 Laskar. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.