MOJ
eISSN: 2575-9094


Research Article Volume 1 Issue 3
1Key Laboratory at Universities of Education Department of Xinjiang Uygur Autonomous Region, China
2College of Food Engineering and Biotechnology, Tianjin University of Science and Technology, China
Correspondence: Dr.Yan Zhang, Key Laboratory of Food Nutrition and Safety, Ministry of Education Tianjin University of Science and Technology, No. 29, 13th Avenue, Tianjin Economic and Technological Development Area (TEDA), Tianjin, 300457, China, Tel 8613752353154
Received: July 23, 2017 | Published: September 14, 2017
Citation: Brad K, Zhang Y. Extraction and purification of fructus sophorae genistein and physicochemical properties of its complex with lecithin. MOJ Drug Des Develop Ther. 2017;1(3):89-92. DOI: 10.15406/mojddt.2017.01.00014
Genistein was extracted from Sophoricoside by acid hydrolysis and recrystallization. In order to improve the water solubility and the bioavailability of genistein, the complex of genistein and lecithin was prepared and its physicochemical properties were characterized in this study. Based on UV analysis, there was no significant difference between the physical mixture and the complex, while the result of FT-IR analysis indicated the characteristic absorption peaks of genistein were subdued by the absorption peaks of lecithin. SEM showed the irregular form of the complex. DSC thermo grams of the complex mainly showed the disappearing of characteristic endothermal peaks of genistein, while X-diffractograms showed that the irregularity of the complex. The data of UV, XRD, IR and DSC were in consistent with each other. The synthetic process didn't break the conjugated structure of genistein. The complex is held together by hydrogen bonding and van der Waals force. It possesses new physical and chemical characteristics. The industrial application of genistein can be augmented by increasing of the bioavialability.
Keywords: genistein, lecithin, complex, physicochemical characteristics
ALT, alanine aminotransferase; LG, the complex of lecithin and genistein by synthesis; PMLG, the physical mixture of lecithin and genistein; UV, ultraviolet; IR, infrared radiation; FT-IR, fourier transform infrared; NMR, nuclear magnetic resonance; SEM, scanning electron microscopy; DSC, differential scanning calorimetry; XRD, x-ray diffractometry
Fructus Sophorae, a kind of traditional Chinese herb, contains a variety of chemical components such as flavonoids, which are the components with the highest proportion. Fructus Sophorae possesses various functions including the efficient ability to decrease ALT content, anti-fertility, anti-cancer and anti-tumor activity.1,2 As a kind of medicine with long history and vast plantation coverage, its medicinal value has aroused great attention in modern medicine development. The flavonoids in Fructus Sophorae are mainly composed of Fructus Sophorae glycosides and genistein. Fructus Sophorae glycosides can be transformed into genistein by acid hydrolysis.3,4 Genistein can be used as a medicine for the prevention of osteoporosis. It also possesses various activities such as anticancer, reducing blood fat, anti-oxidation and estrogen effect.5–7 Research reports also indicated that it can be used to improve certain symptoms of menopause women. Since the solubility of genistein is low in water, thus its bioavilability in oral application in functional food and drugs by oral administration is greatly limited solubility. Lecithin molecule contains both the hydrophobic fatty acid ester and the hydrophilic phosphate group. The oxygen atoms existed in the hydroxyl phosphorus atoms of lecithin have strong tendency to obtain electron, while the nitrogen atoms in the groups tend to be strong electron donors. As a result, it can react with compounds with certain structures to form complexes under certain conditions. An additional experiment in this study showed that the equilibrium solubility of genistein was 2.36±1μg/ml in water and the complex was 167.21±11μg/ml (The saturated aqueous solution of genistein and compound were prepared at room temperature, respectively ,and the equilibrium solubility in water was obtained by external standard method.). Many studies have shown that complexes formed with lecithin have better bioavailability compared to the original compounds due to its improved physical and chemical properties.8–10 With the advantage of simple preparation method and low cost, this method can be used to synthetize a wide variety of complexes with higher bioavailability. In this study, sophoricoside and Fructus Sophorae glycosides were extracted from Fructus Sophorae. Then Fructus Sophorae genistein was obtained by acid hydrolysis and recrystallization (95% ethanol) from the mixture. Next, genistein and lecithin complex were synthetize and its physical and chemical properties were studied.
Materials and chemicals
The preparation and purification of genistein: Fructus Sophorae was collected in Yili, Xinjiang, China in August 2015. The material was confirmed by Prof. Korbanjhon Brad in the School of Chemistry & Environmental Engineering, Yili Normal University and was preserved with the identification number of YLNU2015082705 in the specimen preservation site of the university. The lecithin was provided by Sangon Biotech. All the chemicals and solvents used in the experiment were of analytically pure agent.
Fructus Sophorae was crushed into powder before being refluxed in 10 volumes of 90% ethanol for 2h (Reflux temperature was 79˚C). This process was repeated for two times. Then the extract was filtered using filter paper after cooling. The filtrate was initially washed by 2 volumes of waterafter ethanol removal. The precipitate was collected and dried. For further purification, the extraction was refluxed by 10 volumes of dichloromethane for an hour to remove impurities with low polarity (Reflux temperature was 40˚C). Again, the precipitate was collected and dried. Then a solution consisted of 5% HCl, 40% ethanol and 55% water was used to acid hydrolysis of the precipitate by hot reflux for 5 hours (Reflux temperature was 84˚C). Finally, the white crystal of genistein was obtained by recrystallization (95% ethanol), water washing and lyophilization.
The Identification of genistein: The structural characteristics of genistein were identified by various methods such as UV, FT-IR, NMR, mass spectrum and DSC analysis in order to certify that the obtained crystal is genistein with high purity.
The synthesis of the complex of lecithin and genistein (LG): 100 mg of genistein and 250 mg of lecithin were dissolved in 50 mL tetrahydrofuran before magnetic stirred at room temperature for 3 hours. Then they were filtrated through filter paper. Then the yellow substance of LG was obtained from the filtration after the solvent was removed by vacuum concentration.
The preparation of the physical mixture of lecithin and genistein (PMLG): 100 mg of genistein were fully mixed with 250 mg of lecithin to obtain the physical mixture.
The UV and FT-IR analyses
1 mg of lecithin, genistein, LG and PMLG were dissolved in 10 mL of methanol separately for the UV spectra analysis. The UV spectra analysis was conducted under the wavelength of 220 to 500 nm on a scanning UV spectrophotometer (UV-2500PC, Shimadzu, Japan).
The sample of the FT-IR analysis was prepared by 1 mg sample and 150 mg dried KBr. Briefly, after being fully mixed and crushed to powder, the powder was pressed into a 1 mm thick disk for the analysis. Fourier transform IR spectrophotometer (VECTOR22, Bruker, Germany) was used to carry out this experiment. The measuring range was set as 4000-500 cm-1. The data was recorded and processed by the OPUS software (Bruker, Germany) supplied with the instrument.
Scanning electron microscopy (SEM): The testing samples of lecithin, genistein, LG and PMLG were adhered on a copper testing stub before a layer of gold was evenly sputtered on them. The micrographs were obtained with an accelerating potential of 10 kV under low vacuum. A SU1510 (Hitachi, Japan) scanning electron microscope was used to observe the micro-structure of the samples.
Differential scanning calorimetry (DSC): The samples sealed in the aluminum crimp cell were heated at the speed of 10˚C/min from 30 to 300˚C in an atmosphere of nitrogen (50 ml/min using DSC equipment (DSC60, Shimadzu, Japan). The data were recorded and processed by software (Shimadzu, Japan).
X-ray diffractometry (XRD): 100 mg olecithin, genistein, LG and PMLG were used to conduct the XRD experiment, respectively. Specifically, monochromatic Cu Ka radiation (wavelength =1.54056 A˚) produced by a D8 Advance X ray diffractometer (XRD-6100, Shimadzu, Japan) was used to carry out the experiment. In preparation of the experiment, the powdery samples were packed tightly in a rectangular aluminum cell prior. The tube voltage was set as 40 kV under the tube current of 40 mA. The scanning regions of the diffraction angle (2θ) were wetted between 5 -70˚C with the scanning speed of 4˚C/ min. The radiation was detected with a proportional detector.
The identification of structural characteristics
UV, FT-IR, NMR, mass spectrum and DSC analysis were conducted to identify the structural characteristics of genistein
Property: Transparent acicular crystal; m.p.302-305˚C;UV λmax (nm): 262,332; IR (kBr, νmax, cm-1): 3408,1652, 1614, 1565, 1517, 1503, 1308, 1044, 790; ESI-MS m/z:269[M-H]; 1H-NMR (DMSOd6,400 MHz) δ: 12.97(C5-OH), 10.91(C7-OH), 9.61(C4′-OH), 8.33(s, 1H, C2-H), 7.38(d, 2H, C2′, 6′-H), 6.82(d, 2H, C3′, 5′-H), 6.39 (s, 1H, C8-H), 6.23(s, 1H, C6-H). 13C-NMR (DMSO-d6,400 MHz)δ:93.554(C-8), 98.849(C-6), 104.343(C-10), 114.939(C-3′, 5′), 121.083(C-3 ), 122.153 (C-1′), 130.058 (C-2′, 6′),153.906 (C-2), 157.303 (C-9), 157.470 (C-4′),161.882(C-5), 164.161(C-7), 180.104(C-4). These data were in consistent with the references, thus it can be identified that the compound is genistein [8-10].
Spectra analyses
The UV spectra of lecithin, genistein, LG and PMLG were shown in Figure 1. No difference was observed between the spectrum of LG and PMLG. The same as that of genistein, characteristic absorption peaks were observed at 262 and 332 nm in these two samples. This suggested that no change happened in the absorption generated and conjugated structures existed in the structure of genistein that cause these absorption peaks. It could be observed from the infrared spectra of these four (Figure 2). Judging from the similarity of characteristic absorption peaks of LG and PMLG, these two figures were resulted from the pilling up of lecithin and formonetin. Meanwhile, some characteristic absorption peaks of genistein observed between 500cm-1~1750cm-1 were subdued by lecithin and thus this characteristic didn't occur in the absorption peak of LG. Combined with the fact that no new absorption peak was observed in the figure of LG, it can be concluded that no conjugated bond was formed during the synthesis of LG and the conjugated structure of both lecithin and genistein remained intact in the complex.
SEM analysis
The SEM images of lecithin, genistein, LG and PMLG were given in Figure 3. As seen from Figure 3, the surface of lecithin showed irregular structure, while genistein appeared as acicular crystals. Both the irregular structure of lecithin and the acicular crystals of genistein were observed in the sample of PMLG. This indicated that lecithin and genistein in PMLG were simply mixed together. The SEM image of LG showed that the structure of lecithin is irregular. And this indicated that genistein was successfully incubated in the structure of lecithin.



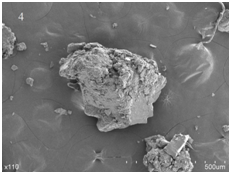
Figure 3 Scanning electron micrographs of lecithin (1), genistein (2), their physical mixture (3) and complex (4).
Thermal characteristics
The DSC thermo grams of lecithin, genistein, LG and PMLG were presented in Figure 4. The thermo gram of genistein showed an endothermal peak (onset temperature at around 302˚C). It was the temperature that genistein started to melt. The thermo gram of PMLG showed a simply pilling up of the thermo gram of lecithin and genistein. The fact that its endothermal peak is not significant may lead to the low content of genistein in the physical mixture. The endothermal peak of lecithin disappeared in the thermo grams of LG. This resulted in the similarity of the thermo grams of LG and lecithin. The result suggested that genistein was evenly dispersed in the structure of lecithin and that the two maybe connected by Hydrogen bonding and van der Waals force.
XRD analysis
The powder X-ray diffraction patterns of genistein, lecithin, LG and PMLG were provided in Figure 5. Sharp crystalline peaks indicate the presence of crystalline organic molecule. By contrast, amorphous property without the crystalline peaks was observed in the diffraction pattern of lecithin. Due to the effect of lecithin, the intensity of peaks in the PMLG was subdued, while the peaks were completely disappeared in the LG. The result suggested that genistein was evenly dispersed in the structure of lecithin and that it existed in a kind of irregular form.
Genistein with high purity in the form of transparent acicular crystal was obtained by solvent extraction, refluxing and recrystallization. Various methods such as NMR, Mass spectrometry, UV and DSC analyses were used to identify the transparent acicular crystal to be genistein.
A series of ratios of genistein to lecithin had been tried to synthesize the LG. The optimum ratio was found to be 1:2.5 (m/m). The mole ratio is close to 1:1 when the Mw of genistein and lecithin is regarded as 270 and 750, respectively. LG was synthesized in tetrahydrofuran. It showed the better solubility compared to genistein which is in consistence with the reports of several other complexes. The UV data indicated that the conjugated groups of genistein which caused the absorption peaks remained unchanged. Judging from the similarity of characteristic absorption peaks of LG and PMLG, these two figures were resulted from the pilling up effect of the figure of lecithin and genistein.Meanwhile, some characteristic absorption peaks of genistein were subdued by that of lecithin and thus did not occur in the absorption peak of LG. This indicated that the basic chemical structure of both genistein and lecithin remained unchanged in LG and PMLG.
Results of the SEM analysis showed that the surface of lecithin was irregular structure, while genistein was acicular crystals. The figure of PMLG illustrated a mixture of lecithin and genistein, while that of LG showed the irregular structure of lecithin.
The thermo gram of genistein showed an endothermal peak at around 302˚C. No obvious endothermal peak was observed in that of lecithin and LG, which is attributed to the polarity end of genistein, lecithin were interacted with each other and that the regularity decreased between the fatty hydrocarbon chain of phospholipid molecules in lecithin with the interaction of genistein.
In the XRD analysis, sharp crystalline peaks indicated the presence of crystalline organic molecule of genistein. By contrast, a wide peak was observed in the diffraction pattern of lecithin. This indicated the amorphous property without the crystalline peaks. The crystalline peaks were also showed in the figure of PMLG. Although its intensity influence of lecithin was subdued, the peaks were completely disappeared in the LG. This indicated that the crystalline characteristics of genistein were submitted by lecithin. This effect was supposed to increase with the increasing ratio of lecithin. Judging from the figure of LG, the crystalline peaks were almost disappeared. Only small amount of the subdued peaks were observed, which showed the irregular characteristics of lecithin. This indicated that genistein might be relevant to lecithin and the effect undermined the crystalline characteristics of genistein.11-13
The polarity end of lecithin and genistein were relevant to each other in tetrahydrofuran by Hydrogen bonding and van der Waals force. The synthesized LG possesses with new characteristics, showing significant change compared with the original chemicals as well as the physical mixture.
The data of UV, XRD, IR and DSC were in consistent with each other. These data suggested that the characteristics of LG were significantly different from that of PMLG, lecithin and genistein. The results also showed that neither was the conjugated groups in genistein changed, nor formed during the synthesis process. Genistein was only evenly dispersed in the structure of lecithin and that the complex existed in the form of irregularity. The two were connected by Hydrogen bonding and van der Waals force. The complex might enhance the potential value in the industrial application of genistein.
The financial support provided by the National Science Foundation of China (31401663) is greatly appreciated.
The author declares no conflict of interest.

©2017 Brad, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.