MOJ
eISSN: 2641-9297


Research Article Volume 1 Issue 4
Department of Medical Biology, Siberian Federal University, Russia
Correspondence: Nataliya Menzyanova, Associate Professor, Department of Medical Biology, Siberian Federal University, Svobodnyi Ave. 79, Krasnoyarsk, 660041, Russia, Tel 8 983 501 88 80
Received: July 30, 2018 | Published: August 17, 2018
Citation: Shishatskaya E, Menzyanova N, Pyatina S, et al. Biological activity of humic acid preparations in the form of nanoscale particles in the hydroponic culture of Triticum aestivum seedlings. MOJ Curr Res & Rev. 2018;1(4):180-188. DOI: 10.15406/mojcrr.2018.01.00029
In the hydroponic culture of T. aestivum, the biological activity of humic acid (HA) preparations in the form of nanoparticles of different sizes were studied. When cultured on media containing HA preparations, the content of carbonylated proteins, malonic aldehyde and proline, which are the markers of induced oxidative stress, increased in the roots of the 2-day seedlings. The oxidative stress induced by HA was accompanied by an increase in the number of free border cells and the size of the mucilage cup in the root apex of the 2-day seedlings, but destructive changes in the root apex and root growth inhibition were not observed. The effects of HA preparations were dose-dependent and varied significantly, depending on the size of the HA nanoparticles.
Keywords: hydroponic culture T. aestivum, humic acids, border cells, oxidative stress, root apex, rhizosphere, proline, MDA, carbonylated proteins
Humic acids (HA) are water-soluble organic compounds that are formed as a result of biodegradation of plant and animal remains with the participation of soil microorganisms.1 HA are used as plant growth stimulants2–4 and as agents that increase the resistance of plants to various stress factors5,6 and nematode infections.7 Numerous experimental data suggest that the biological effects of HA are implemented through the restructuring of transcription and posttranslational events, which leads to significant changes in metabolism, development and growth of plants.8,9 However, the signaling pathways and effectors targets of HA have been studied piecemeal and there is no complex model of biotropic effects of HA.8 In most cases, the effects of HA are associated with hormone- and ROS-signaling.10–12 The interaction of these two signaling systems determines the stimulating effect of HA on the growth of seedlings and the root system. However, the fine regulatory interactions of these signaling systems can vary depending on the type and age of the plants, cultivation conditions and also on the concentration and methods of obtaining HA.13
Thus, HA, obtained from the soil, stimulate the carbon metabolism14 and HA, isolated from vermicompost, stimulate the development of lateral roots.15 Lignite HA (with a lower molecular weight) are more effective as root growth stimulants than HA obtained from the manure of cattle.10 It is shown that such parameters as lability and stability can influence the biological activity of HA preparations.11 Under conditions of mass cultivation, the variability of the effectiveness of HA preparations can significantly affect the yield and cost of production. This determines the practical importance of studying the biological activity of HA preparations in model systems for predicting their effectiveness in conditions of agriculture. In this connection, the biological activity of HA preparations obtained by the electrochemical method from aqueous peat suspensions in the hydroponic culture of T.aestivum seedlings was studied in this paper.
Grain germination
T.aestivum grains were washed for 5-6 hours under running water and soaked for 24hours in distilled water at room temperature. Seeded grains were placed in Petri dishes, 50pcs into each dish. In the control variant, 7ml of distilled water was added to the plates. In the experimental variants, 7ml of solutions of humic acid preparations with a concentration of 5, 25 and 100mg/L were added. HA were obtained from peat (Kedrovsky deposit, transitional type of peat) by electrochemical method. Stable nano-sized fractions of humic acid particles were obtained by various regimes of pulsed electrochemical action. The concentration of humic acids was determined by the method described by Lamar et al.,16 Grain was germinated at room temperature with round-the-clock illumination. In 2-day-old seedlings the length of the main root was determined.
Estimation of the population size of border cells (BCs)
The BCs were washed off the surfaces of the root apex in the distilled water on a magnetic stirrer. For this, 20 main roots were cut off, the root apexes were leveled, clamped with tweezers and washed in 1ml of distilled water on a magnetic stirrer for 1min. The final cell suspension was centrifuged at 3000g, 10min. The supernatant after centrifugation was an aqueous solution of the mucilage cup components (proteins, polysaccharides, etc.). The supernatant was collected to determine the total protein by Lowry.17 The BCs pellet was suspended in the distilled water and centrifuged again 3000g, 10min. The BC was fixed in 2.5% glutaraldehyde for 1hour. After fixation the cells were washed twice with distilled water and stained with 0.01% methylene blue. In the cell suspension the total number of cells (single cells, cell strings, and cell layers) and the number of single cells in % were determined.
Preparation of semi-thin sections
Main roots of 2-day-old seedlings were cut off. The roots were fixed in 2.5% glutaraldehyde on a phosphate-salt solution (PBS, pH 7.0) for 3h. The roots were washed in the distilled water and re-fixed in 1% OsO4, 30 min. After the second fixation, the roots were washed with distilled water, the samples were dried in ascending concentration alcohols and acetone, and poured into epon (EMbed 812 Kit). Semi-thin sections were stained with 1% basic fuchsin and 0.1% toluidine blue in 2.5% sodium carbonate (pH 11.1). Epone was applied to the slises, covered with coverslips and after polymerization the preparations were analyzed under a light microscope.
Preparation of samples for scanning electron microscopy
The samples were fixed and dewatered as described in 2.3 and analyzed using a TM-3000 scanning microscope (Japan).
Determination of the content of carbonylated proteins, MDA and proline
Fragments of the main roots with root apices 1 cm long were cut off in 2-day-old seedlings. The resulting root biomass was homogenized in 0.05M Tris-HCl buffer, pH=7.4, at T=4°C. The homogenates were centrifuged to remove coarse debris at 5000g, 45min, at T=4°C. The supernatant was collected and used to determine the carbonylated protein content by the method of Carty et al.,18, malonic dialdehyde by the method of Bailly et al.19 and proline by the method of Bates et al.20 The content of carbonylated proteins, malonic dialdehyde and proline was recalculated on the basis of 1 mg of protein in root homogenate. Statistical processing of the results was carried out using the Student-test.
Effect of HA preparations on the root length and the population of BC in the root apex of T. aestivum seedlings
The preparation of HA with small particles (average size 70nm) in the concentrations studied did not affect the root length of the 2-day seedlings. The preparation of HA with big particles (average size 300nm) stimulated root growth only at a concentration of 100mg/L: the root length was 1.4 times greater than in the control variant (Figure 1). Thus, significant stimulation of root seedling growth on media with HA preparations in the form of nanosized particles was not observed. The absence of pronounced stimulating effects (as well as the lack of inhibition effects) can be due to the following reasons:
HA in the form of nanoscale particles do not have biological activity
In the early stages of germination, effector systems have not yet been formed in the seedlinging root, the activity of which may depend on HA.
For example, the processes of cell extension, on which the extension of the root of the seedling depends on the early stages of germination, may not depend on HA. In this connection, at the next stage of the work, the effects of HA in the form of nanoscale particles were estimated at the rhizosphere level. The rhizosphere is one of the potential effector targets of standard preparations of HA. BCs of the root apex play an important role in the formation of the rhizosphere system of the developing seedling. The BCs on the surface of the root apex are detected at the earliest stages of root seedlinging; probably, this population of cells is formed even under the cholorise (Figure 2). The BCs are a morphologically heterogeneous population of cells (Figure 3). At the upper part of the apex rounded cells are localized. The cells extend and become rod-like in shape as they move away from the upper part. On microphotographs, it is possible to observe various phases and features of separating the BC from the root apex. Round and oval cells on the apex "go" into the matrix of the mucilage cup “one by one”. On the lateral surfaces elongated cells, and especially rod-shaped cells, are peeled off in groups in the form of long chains and layers: the BCs lose contact with the apex surface, but retain contacts with neighboring cells in the separating monolayer. Free single BCs and BC aggregates are held near the root apex by the matrix of the mucilage cup formed by the excretion products of the BCs (Figure 3). It is known that BCs actively excrete more than 100 different proteins into the rhizosphere.21,22 The size of the mucilage cup can be inferred by the size of the "scatter" of free BCs. Small mucilage cups with a small BC population are observed in the control variant (germination on distilled water) (Figure 3A). When cultured on media with HA preparations, the dimensions of the mucilage cups significantly increase: free BCs can be located at a distance of 500-600μm from the surface of the root apex (Figure 3B–3D). At the same time, the number of free BCs in the mucilage cup increases significantly.
For cultivation options with HA preparations, one can distinguish 3 morphotypes of the rhizosphere space around the apex (Figures 3B–3D):
Effects of HA preparations were detected only in the BC population. The analysis of serial semi-thin sections of root apex revealed no destructive changes in the cytoarchitectonics of the apical meristem of seedlings that were cultured on media with HA preparations (for all HA concentrations studied in the form of big particles and small particles, Figure 4). At the same time, a well-developed mucilage cup with numerous BCs, which lost contact with the apex surface (Figure 4D-I & Figure 5B) was revealed on the transverse semi-thin sections. In control (distilled water), the mucilage cup was identified along the perimeter of the cut as a narrow colored ring (Figure 4A–4C & Figure 5A). The analysis of root apexes of seedlings with the help of scanning electron microscopy showed that the surface of the epiblema (rhizoderma) when cultured on media with HA preparations remained intact, without visible changes (Figure 6B–6D). In the areas of the epiblema surface, from which the BCs were separated, there were also no visible destructive changes (Figure 7B–7D). To estimate the number of BCs that have already lost contact with the surface of the root apex, the mucilage cup was washed with a magnetic stirrer and the resulting cell population was analyzed. It was shown that after "dissolving" the mucilage cup contacts between the cells are preserved: in the cell suspension, along with single cells, cell chains and cell layers were observed (Figure 8). In control, the number of single cells was 47% of the total number of BCs flushed from the surface of the root apex. Entering into the culture medium the preparation of HA with big particles in a concentration of 100mg/L led to an increase in the number of single cell 1.4 times, and for the preparation of HA with small particles, in the same concentration, on the contrary, there was a decrease in the number of single BCs 1.6 times, compared with the control (Figure 9). That is, in a medium with HA with big particles, the BCs went to the rhizosphere mainly in the form of single cells (64% of single cells), and on a medium with small particles they went to the rhizosphere in the main form of cell layers and cell chains (71% of all free BCs). HA not only influenced the "way" of the transition of the BCs to the rhizosphere, but also caused a dose-dependent increase in BC numbers in the rhizosphere. Thus, HA in the concentration of 100mg/L in the form of big particles increased the number of BCs 5 times, and the shape of small particles - 11 times, compared with the control variant (Figure 10).
Active flushing allowed not only to collect the BCs, but also to obtain a solution of the mucilage cup components. Determination of the total protein in these solutions showed that the adding of HA in the culture medium led to a dose-dependent increase in the protein content in the rhizosphere of the 2-day seedlings (Figure 11). Since the components of the mucilage cup (including proteins) are excreted by the BCs, the revealed patterns can be related both to the increase in the number of BCs and to the increase in their secretory activity. In order to estimate the effect of HA on the excretory activity of the BCs in the first approximation, the calculated parameter was used for the ratio "protein content in one mucilage cup" / "number of BCs per root apex". This ratio was defined as the excretory activity of the BCs. It was shown that HA preparations significantly increased excretory activity of BCs, but the dose-dependent dynamics of excretory activity differed significantly for HA preparations with big particles and small particles (Figure 12). Thus, HA with big particles increased the excretory activity of BCs at a concentration of 5mg/L, but a further increase in HA concentration did not lead to significant changes in excretory activity. For HA with small particles, the maximum stimulating effect was observed for a concentration of 25mg/L, and for a concentration of 100mg/L there was a sharp decrease in the excretory activity of BCs. The obtained results indicate that HA in the form of nanosized particles:
Increase the activity of exfoliation of BCs from the surface of the root apex and, as a result, the number of free BCs in the mucilage cup; the separation of BCs from the apex surface is observed shortly after the rupture of the coleorhiza (Figure 2C, 2D);
Affect the excretory activity of the BCs
Rhizospheric effects of HA are dose-dependent and differ significantly for HA preparations with big particles and small particles.
Another effector system of HA is redox homeostasis: biological activity of HA can be realized through the induction of mild oxidative stress. In this connection at the next stage of the work, the influence of HA in the form of nanoparticles on the content of carbonylated proteins, MDA and proline, which are the markers of oxidative stress, was evaluated.
The effect of HC preparations on the content of MDA, carbonylated proteins (CPs) and proline in the roots of the 2-day-old seedlings T. aestivum
When cultivated on media with preparations of HA with big particles at a concentration of 25mg/L and 100 mg/L in the roots of 2-day-old seedlings, the content of CPs increased 2 and 5 times, respectively, compared to the control (Figure 13a). When cultured on media with different concentrations of HA preparations with big particles, the MDA content in the roots of the seedlings did not differ significantly from the control (Figure 13B). Comparison of the dynamics of the content of CPs and MDA suggests that on medium with preparations of HA with big particles, a significant part of the formed MDA is included in the oxidative modifications of proteins and the TBA-reactive pool remains within the values typical for the control variant (Figure 13). In contrast to HA with big particles, under the influence of preparations of HA with small particles, the MDA content in the roots of the seedlings increased 2-fold (concentrations of 25mg and 100mg/L). An increase in the content of CPs in the roots was noted only for a concentration of 100mg/ml (Figure 13A, 13B). The increase in the content of CPs and MDA was accompanied by a dose-dependent increase in proline content in the roots of seedlings (Figure 14). At a concentration of 25mg/L, both HA preparations increased the proline content by 1.4 times. At a concentration of 100mg/L, preparations of HA with small particles led to an increase in proline content by 3 times, and preparations of HA with big particles by 2 times (compared to control). An increase in the content of CPs, MDA and proline in the roots of seedlings during cultivation on media with HA suggests that HA in the form of nanoparticles are inductors of oxidative stress. The effects of HA preparations depend on the concentration and particle size. It should be noted that changes in proline, MDA, and CB content induced by HA preparations were not accompanied by inhibition of root growth.
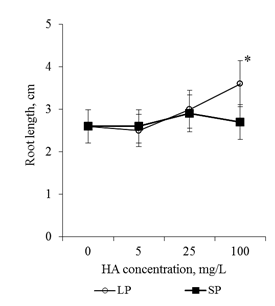
Figure 1 The length of the main root of the 2-day old seedlings T.aestivum after germination on media with HA preparations in the form of man-sized particles.
*- the value is significantly different from the control variant (p<0.05). LP-large particles (300nm), SP-small particles (70nm).
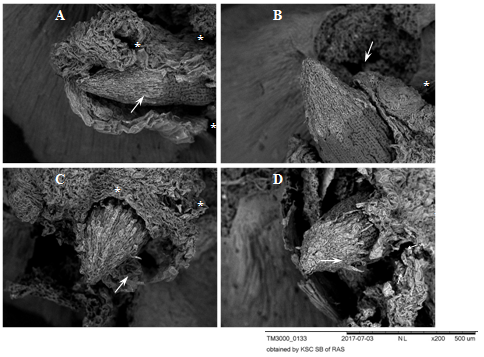
Figure 2 Root apexes of 2-day-old seedlings T.aestivum, germination for 8-10 hr. Scanning electron microscopy.
A, B: control variant (germination on distilled water); C, D: germination on media with HA preparations in the form of nanosized particles. The apexes are surrounded by a ruptured coleorhiza (white asterisks). The apexes are covered with a BC: caps with light gray cells with rounded nuclei projecting on the surface (white arrows). In the control variant (A, B), the BC is closely adhered to the surface of the apex. In the experimental variants (C, D), the BC layer (C) is loosened and the cells peel off from the surface (D, at the lower edge of the BC cap, short thin arrows).
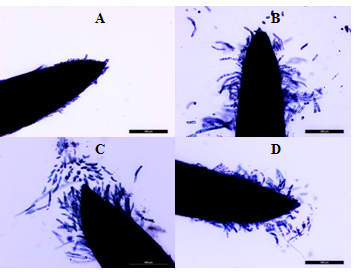
Figure 3 Root apexes of 2-day-old seedlings T. aestivum. Staining with trypan blue.
A: cultivation on the distilled water. B, C, D: germination on media with HA preparations in the form of nanosized particles; B: active lateral exfoliation of chains of elongated, rod-shaped cells. From the BCs chains around the apex, a "crown" is formed, which keeps contact with the root apex (due to the cells at the base of the " crown "); C: active separation of the BC from the top of the apex (single cells) and from the side surface of the apex; D: the surface of the apex is covered with loose cells, many of which still retain contact with the surface of the apex. Bar = 400µm.
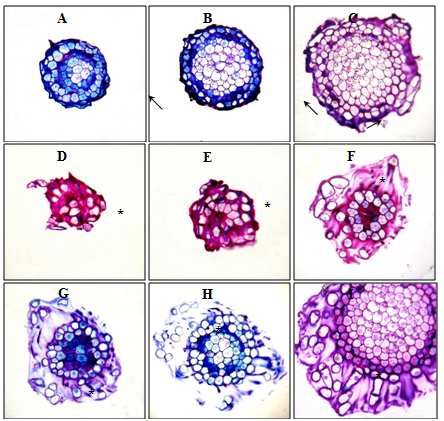
Figure 4 Root apexes of 2-day-old seedlings T. aestivum, serial transverse semi-thin sections, from the top apex to the base, staining with 1% basic fuchsin and 0.1% toluidine blue.
A, B, C: root apex in the control (on distilled water). Along the perimeter of the cuts, a narrow ring of the mucilage cup of violet color (indicated by arrows) is localized; D–I: root apex on medium with HA preparations in the form of nanosized particles. Apex is surrounded by a well-developed mucilage cup: a substance colored in purple color, in which there are BCs that have lost contact with the apex surface (BCs are marked with asterisks). The mucilage cup covers the top and side surface of the apex; D, E: cut the top of the mucilage cup with free BCs, without cutting the root apex. x400
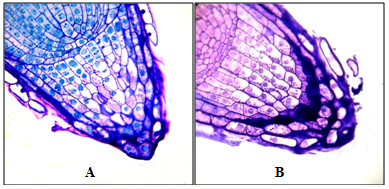
Figure 5 Root apexes of 2-day-old seedlings T. aestivum, Semi-thin longitudinal sections. Staining with 1% basic fuchsin and 0.1% toluidine blue. The plane of the cut passes above the middle of the apex.
A: apex in the control variant (on distilled water); B: apex on medium with HA preparations in the form of nanosized particles. At the top of the cut are BCs that have lost contact with the side surface of the apex, but are held by the mucilage cup (the BCs are marked with white asterisks). x400
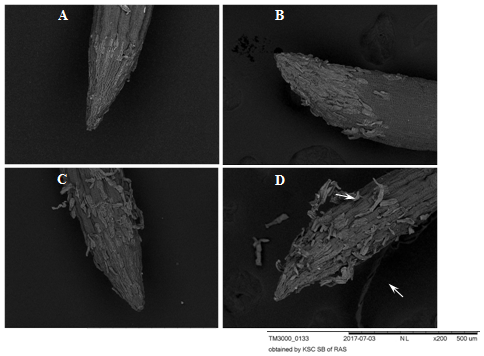
Figure 6 Root apexes of 2-day-old seedlings T.aestivum, Scanning electron microscopy.
A: control variant, (on disilled water). The BCs are firmly pressed against the surface of the apex; B, C, D: germination on the medium with HA preparations in the form of nanosized particles; В: a loose layer of PCs that have not lost their connection with apex; C: single cells separate from the apex surface; D: BCs are separated from the apex surface by layers (indicated by arrows). The surface of the epiblema (rhizoderm) in the control and in the experimental variants is the same (dark gray corrugated areas of the root surface). Under the separating BCs, the epiblema surface is intact.
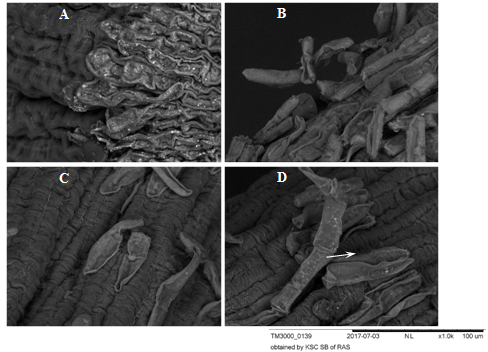
Figure 7 BCs of root apexes of 2-day-old seedlings T.aestivum, Scanning electron microscopy.
A: control variant (on distilled water). The BCs are pressed against the apex surface and contact each other forming a monolayer; B, C, D: germination on the medium with HA preparations in the form of nanosized particles; В: a loose layer of BC on the apex surface, contacts in the BC layer are already broken, but there are points of "contact" with the surface of the apex; C: single BCs separated from the surface of the apex; D: the BC is separated from the apex surface by a chain (indicated by an arrow).
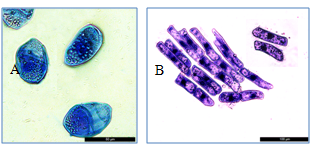
Figure 8 BCs in the suspension after active flushing.
A: oval cells. Starch granules are seen in the cytoplasm. Bar = 50µm; B: rod-shaped cells, aggregate in the form of a monolayer and two single cells. Bar =100µm.
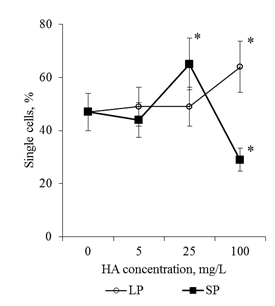
Figure 9 The number of single cells in the BCs suspension. BCs were collected by active flushing from the root apexes of the 2-day seedlings T.aestivum after germination on media with HA in nanoparticle form.
*- the value is significantly different from the control variant (p <0.05). LP: large particles (300nm); SP:small particles (70nm).
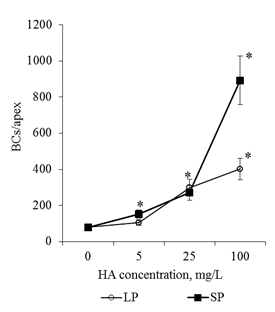
Figure 10 The number of BCs in the root apex of the 2-day seedlings T.aestivum after germination on media with HA in nanoparticle form. The cells were prepared by actively flushing from root apexes.
*- the value is significantly different from the control variant (p<0.05). LP: large particles (300nm); SP: small particles(70nm).
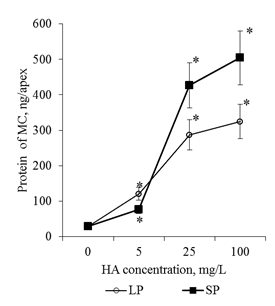
Figure 11Protein content in the mucilage cup(MC) of the root apex (ng/apex) of the 2-day seedlings T.aestivum after germination on media with HA in n anoparticle form.
*- the value is significantly different from the control variant (p<0.05). LP: large particles (300nm); SP: small particles(70nm).
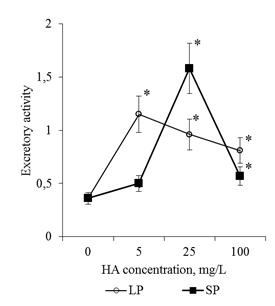
Figure 12 Excretory activity of BCs of 2-day seedlings of T. aestivum after germination on media with HA in nanoparticle form. Excretory activity was evaluated as the ratio of the amount of protein in the apex mucilage cup and the number of BCs in apex.
*- the value is significantly different from the control variant (p<0.05). LP: large particles (300nm), SP: small particles (70nm).
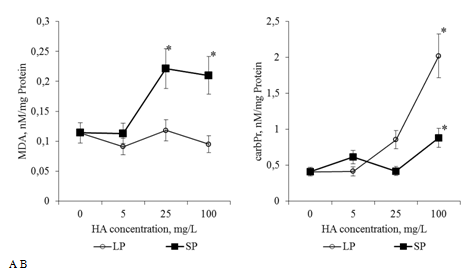
Figure 13 The content of MDA: A and carbonylated proteins (carbPr): B (nM/mg protein) in the root apexes of 2-day seedlings T.aestivum after germination on media with HA in nanoparticle form.
*- the value is significantly different from the control variant (p<0.05). LP: large particles (300nm); SP: small particles (70nm).
In the literature, the biological activity of HA are often explained by their effect on the rhizospheric metabolism.11,12,23 The rhizosphere is formed in the process of root excretion and plays an important role in the adaptation processes of the developing root of the seedling and adult plant to various biotic and abiotic factors of the environment.24–28 It is shown that the most important structural-functional element of the rhizosphere system is the BCs. 29–32 BCs are a specific population of somatic metabolically active cells. At different stages of differentiation, BCs can realize their functions in conditions of physical contact with root apex, and after loss of physical contact with root apex - in mucilage cup, polysaccharide-glycoprotein matrix that surrounds apex and is a product of excretory activity of BCs. Numerous starch granules in the cytoplasm of the BCs testify to the possibility of prolonged autonomic functioning (Figure 8A). HA preparations in the form of nanoscale particles increased the activity of separation of the BCs from the surface of the root apex and transition into the mucilage cup (Figure 3). The separation of BCs is associated with remodeling of the cell walls. It is known that not only enzymes (endo-1,4-mannase, pectin methyl esterase, cellulases) participate in the remodeling of cell walls, but also reactive oxygen species (ROS): a non-enzymatic oxidative modification of the cell wall components contributes significantly to the loosening of the cell walls in the early stages of seedling development.33–36 Since the preparations of HA in the form of nanosized particles induced oxidative stress (increased the content of CPs, MDA, proline, Figure 13 & Figure 14), it can be assumed that an increase in the separation activity of the BCs from the surface of the root apex during germination on media with HA is significant degree is associated with oxidative remodeling of the cell walls. It should be noted that the contribution of the ROS to the processes of remodeling the sections of the cell walls contacting the surface of the root apex and the areas of intercellular contacts between the BCs themselves can be different. So, HA increased the activity of lateral flaking of the BCs from the surface of the root apex, but at the same time BCs contacts were kept with each other (Figure 8).
In the presence of HA preparations in the culture medium, not only the number of free BCs, but also the size of the mucilage cup increased (Figure 3). The dynamics of the calculated excretion activity parameter allows us to believe that an increase in the size of the mucilage cup is associated not only with an increase in the number of BC producers, but also with an increase in the excretory activity of the BCs (Figure 12). (It is known that BCs actively excrete more than 100 different proteins into the rhizosphere21,22). It is possible that the effect of HA preparations on excretory activity of BCs is realized through induction of HA oxidative stress. It is known that inducers of oxidative stress increase the activity of excretion of root exometabolites.26,37
How should the effect of HA preparations on the number of BCs and their excretory activity be treated? Is it the effect of stimulation or protective compensatory-adaptive reaction? There was no pronounced stimulation of root growth in 2-day-old seedlings in the presence of HA preparations, but there was also no inhibition of growth. It is possible that the early formation of a large population of free BCs and the active excretion of exometabolites induced by HA preparations can contribute to the rapid development of the rhizosphere system and, as a result, provide rapid growth and development of the root system, its resistance to various biotic and abiotic factors at later stages of the development of seedlings. Realization of biological effects of HA is associated with the induction of mild oxidative stress: HA increase the production of ROS in plant cells.38,39 ROS signaling, oxidative modification of proteins, lipids and RNA play an important role in the early stages of germination of the grains.40–43 In the germinating grain, the oxidative modification of proteins increases the activity of their proteolytic degradation in various types of proteasome systems and determines the availability of amino acids for inclusion into the synthesis of new proteins44. It can be assumed that the activity of the oxidative modification of proteins largely determines the rate of change in the proteome of the seedling at early stages of its development. HA preparations in the form of nanosized particles led to a dose-dependent increase in the content of CPs (Figure 13A). Based on the literature data, this fact can be considered as evidence of more active synthesis of de novo proteins in seedlings during germination on media with HA. An increase in the content of CPs was accompanied by a dose-dependent increase in MDA (for preparations of HA with small particles, Figure 13B). The level of MDA is a marker of structural and functional rearrangements of membrane cell systems. Oxidative modifications of lipids can affect the stability of membrane rafts, the packing density of fatty acid chains and the microviscosity of the membrane,45,46 induce deformations of the lipid bilayer, pore formation, and transition to the micellar phase.47 These structural changes are considered as regulatory mechanisms that determine the activity of membrane-bound processes and the functional status of the cell. The protein synthesis machinery largely depends on the membrane systems of the cell and the oxidative modifications of the membrane lipids can play an important role in activating the de novo synthesis of proteins and rearrangements of the seedling proteome when cultured on media with HA.
Along with CPs and MDA, proline content is a marker of induced oxidative stress in plant cells. ROS are the mediators of signaling systems that control proline metabolism.48–50 Proline under the conditions of oxidative stress "works" as:
Oxidation of 1 proline molecule in the mitochondria results in the formation of approximately 30 ATP equivalents,56 which are used to implement compensatory adaptive metabolism rearrangements under stress conditions. In this regard, the dose-dependent increase in proline content in the roots of seedlings during cultivation on media with HA (Figure 14) can be considered as evidence of rearrangements of the energy metabolism of the seedling: an increase in the pool of energy equivalents that can be used for the active synthesis of de novo proteins (and other energy-dependent processes). On the other hand, the increase in proline level induced by HA can support the activity of oxidative modifications of proteins and lipids at the "regulatory level" and prevent the development of oxidative modifications that destroy the structural and functional integrity of cellular systems. Oxidation of proline in mitochondria and the formation of ROS equivalents are accompanied by the formation of ROS and secondary activation of the processes of oxidative modification of proteins and lipids and ROS signaling.57 This phenomenon does not allow us to estimate the "magnitude" of the oxidative stress, caused by HA. Discussion of the obtained results allows us to speak about deep rearrangements of the root metabolism of the developing seedlings induced by HA preparations in the form of nanosized particles. It can be assumed that these rearrangements at later stages of seedling development are realized in the pronounced stimulating effects of root system growth and development of the shoots.
In conclusion, it should be noted that the revealed dose-dependent effects of HA preparations were significantly different for small particles and big particles: HA preparations in the form of small particles had a much more pronounced effect on the parameters studied (changes in comparison with the control variant) (Figure 13 & Figure 14). At the same time, the relationship between the parameters (Proline/CP, Proline/MDA, CP/MDA) ratio varied significantly depending on the particle size in the HA preparations (Figure 15A–15C). Peculiarities of the ratio dynamics for Proline/CP, Proline/MDA, CP/MDA suggest that the ROS-dependent signaling can differ significantly for HA particles of different nanoscale classes. It is known that the biological effects of nanosized particles largely depend on the dimensions.58–60 In general, smaller particles are biologically more active than big particles: the level of induced oxidative stress for small particles is higher than for big particles.61,62 It is assumed that the ability of HA to induce oxidative stress in the root system is due to the fact that HA particles can temporarily "clog" pore complexes in the cell walls and thereby induce dose-dependent oxidative stress. Under the influence of root exometabolites, the molecular disintegration of HA particles occurs and the pore complexes are released.23,63 The authors did not study the dependence of oxidative stress on the size of molecular aggregates of HA, but it can be assumed that smaller particles of HA "clog" the pore complexes for a longer time and as a result, the oxidative stress induced by them will be more pronounced than for larger HA particles. The results obtained suggest that the variation in the size of HA particles can be used for the purposeful regulation of their biological activity in the rhizosphere at early stages of germination.
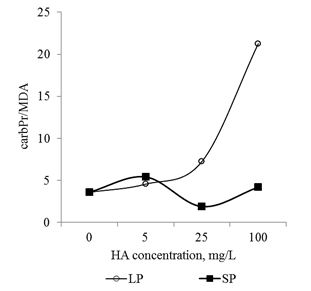
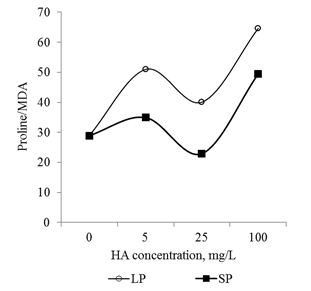
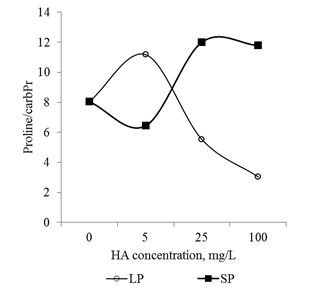
Figure 15 The relationship between the main markers of oxidative stress (proline, carbonylated proteins and MDA) in the root apexes of 2-day seedlings T.aestivum after germination on media with HA in nanoparticle form.
A: ratio of carbonylated protein and MDA content; B: ratio of proline and MDA content; C: ratio of proline and carbonylated protein.
Author declares that there is no conflict of interest.

©2018 Shishatskaya, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.