MOJ
eISSN: 2572-8520


Review Article Volume 3 Issue 5
Faculty of Architectural Engineering, Arab International (Formerly European) University, Syria
Correspondence: Aref A al-Swaidani, Associate Professor, Faculty of Architectural Engineering, Arab International (Formerly European) University, Damascus, Syria
Received: June 07, 2017 | Published: December 7, 2017
Citation: al-Swaidani AM. Modified zinc phosphate coatings: a promising approach to enhance the anti-corrosion properties of reinforcing steel. MOJ Civil Eng.2017;3(5):370-374. DOI: 10.15406/mojce.2017.03.00083
Zinc phosphate conversion coatings are widely used for increasing corrosion resistance and surface preparation for painting. This kind of treatment has recently been introduced in the field of reinforced concrete. However, the disadvantage in the use of such coatings is the existence of pores. Zinc phosphating baths have constantly been developed and modified by additives in order to enhance the coating characteristics including the corrosion resistance and the alkaline stability. These additives and their effects on the resulting coating were reviewed in the current paper. The mechanism of phosphate coating formation, the alkali stability of the coating and the disposal of the phosphating sludge were also briefly reviewed.
Keywords: zinc phosphate coating, reinforcing steel, corrosion of reinforcement, phosphating bath additives, concrete
Reinforcing steel embedded in fresh concrete develops a protective passive layer on its surface. This layer, which is formed as result of high alkalinity of the concrete pore solution (pH~13), consists of γ-Fe2O3 adhering tightly to the steel. As long as that oxide film is present, the steel remains intact.1 However, chloride ions attack and concrete carbonation can destroy the film and, in the presence of H2O and O2 reinforcement corrosion takes place. Chloride ions, which were described by Verbeck2 as the specific and unique destroyer, can be present in concrete either through the use of contaminated aggregate, sea water, brackish water or admixtures containing chlorides.1 In the literature, there are state of the art review studies on numerous methods of protection against reinforcement corrosion.3-8 Applying zinc phosphate coating on reinforcing steel will probably be a vital approach in the future.9-14 The phospahting process has been known for over a century.15 It was extensively used in the automobile and appliance industries.16,17 This treatment primarily provides an inexpensive,17,18 non-toxic,19,20 reasonably hard, highly adherent and electronically non-conducting phosphate coating.15 The insulation properties make an important contribution to the prevention of reinforcement corrosion.14 Further, the phosphate conversion coatings are currently considered eco-friendly.21 However, Phosphate coatings usually contain pores which are pathways for the corrosive electrolyte diffusion into the metal substrate. As a result, the corrosion resistance of the conventional phosphate coating is not high enough. Additives such as Ni2+, Ca2+, Mo2+, Cu2+ and Mn2+ and nanoparticles have been extensively used to produce phosphate coatings with uniform structure, lower porosity and improved corrosion resistance and adhesion properties.22,13,12,23,11,17,24 Being a newly adapted method in the field of reinforcement corrosion protection25-27,13 this paper tries to review some important aspects related to the improvement of corrosion resistance of reinforcement using zinc phosphate coatings modified by metal cat ions or nanoparticles.
Mechanism of zinc phosphate coating formation
Phosphating is essentially an electrochemical process.28 When the steel comes into contact with phosphate solution which is basically a phosphoric acid-based solution, an electrochemical reaction takes place where Fe2+ start to dissolve and release electrons at the anodic site, and the reduction of H+ occurs at the cathodic site. The evolution of hydrogen and consumption of H+ ions results in a local increase of pH at the surface leading to precipitation of zinc phosphate crystals.29 According to Zimmermann,23 Donofrio30 and Kunst,31 the possible reactions taking place in the zinc phosphating bath are as follows:
(1)
(2)
In general, the presence of phosphophyllite is associated with a better corrosion resistance than hopeite, probably due to its enhanced chemical stability relative to alkaline electrolytes.32
Alkaline stability of zinc phosphate coating
Studying the alkaline stability of zinc-phosphated reinforcing steel is of great importance, as it will be embedded in a highly alkaline environment (pH~13). There are few published studies on the alkaline stability of the phosphate coating on reinforcing steel. Literature shows that the alkaline stability of zinc phosphate coatings depends on their chemical composition and crystal structure.33 Hopeite (Zn3(PO4)2.4H2O) has a higher alkaine solubility than phosphophyllite (Zn2Fe(PO4)2.H2O).30,34,35 Thus, alkaline solutions first dissolve the coating composed of hopeite. Then the dissolution continues for layers rich in iron and those mainly composed of phosphophyllite.36 According to the study of Simescu & Idrissi,13 the dissolution of zinc phosphate coating in the alkaline medium (pH~12.5) is accompanied by the formation of hydroxyapatite Ca10(PO4)6(OH)2. Hydroxyapatite formation occurs even in the presence of aggressive chloride ions13 Figure 1. This chemical compound provides an effective protection against reinforcement corrosion and contributes to the reduction in chloride aggressiveness.10 Elshami9 reported that the phosphated steel was more resistant than non-phosphated steel when exposed to alkaline solutions even in the presence of chloride ions. They attributed this behavior to the formation of a dense and protective layer composed of calcium hydroxyzincate (Ca(Zn(OH)3)2.2H2O) which caused passivation of steel in concrete, thus contributing to a decrease in the aggressiveness of chlorides.
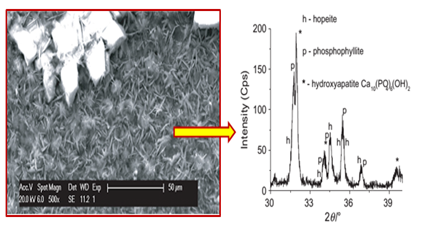
Effects of additives (cations, nanoparticles) on the properties of zinc phosphate coating
Different kinds of additives have been employed in the phosphating bath to improve properties of the phosphate coating. Numerous studies have explored the effects of these additives on the performance of zinc phosphate coatings. Some of these additives are metal cations such as Ni2+, Ca2+, Mn2+, Cu2+ and Mo2+, and nanoparticles like nano-SiO2,11,17,21 nano-TiO2 Shibli & Chacko,37 nano-ZnO38 and nano-CeO2.39 These additives have showed a great improvement to the final coating performance. They were also found to reduce the porosity of the phosphate conversion coating.40
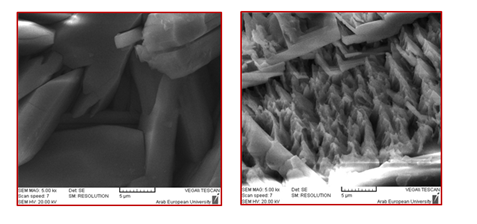
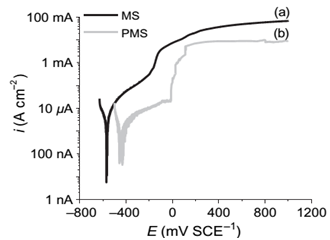
Nickel addition: The addition of Ni2+ ions to the phosphating bath resulted in the modification of the crystalline form, Figure 2.41 As clearly seen in Figure 2, zinc phosphate coating without Ni2+ addition is characterized by plates with sizes up to 30 mm in length and 10 mm in width, whereas the addition of Ni2+ significantly refined the crystals. This grain refinement was also reported in literature.42,43,9,32 In addition, Ni2+ played an important role in the nucleation process, probably through the ion exchange mechanism proposed by Tegehall & Vannerberg.44 Its presence also accelerated the surface reactions during phosphating30 and improved the corrosion resistance of steel.45,23,46,10,14 As shown in Figure 3 & Table 1, after 8 days of immersion in a saturated lime solution containing 35 g.L1 NaCl solution, Ni2+-modified zinc phosphate coating (PMS) showed a lower current density (≈ one-tenth) than mild steel (MS), and decreased the pitting susceptibility by increasing pitting potential at about 160mV compared with MS corrosion currents.10 Further, this addition improved the resistance of phosphate coating against the alkaline environment,13,34 i.e. Ni2+ ions increased the alkaline stability of phosphate layers.34,13 Satoh et al.,47 studied the effects of heavy metal ion addition on zinc phosphating on electro galvanized steels and proposed the substitution of Zn2+ by Ni2+ in hopeite, such as Zn(3-x)Nix(PO4)2.yH2 In a Su & Lin40 study, the addition of Ni2+ in the phosphate solution promoted the impingement of Ni2+, PO43-, and Zn2+. Thereby, the nucleation of the phosphate conversion coating was promoted. Thus, the Ni2+ can accelerate the nucleation of Zn(3-x)Nix(PO4)2.4H2O grains (Figure 2).40 According to Kulinich & Akhta,48 Ni2+ has two main roles in the zinc phospate coating mechanism. First, the rate of increase in local solution pH is limited by the slower kinetics of reactions involving Ni2+ compared to Zn2+, leading to thinner zinc phosphate coatings when Ni2+ is present in the coating solution. Second, most Ni2+ deposition occurs during the later stages of the coating process, by nickel phosphate deposition and formation of a Ni-rich corrosion-resistant oxide (Figure 3 & Table 1).
|
Sample |
Ecorr (mV ECS-1) |
i(µA cm-2) |
Epit (mV ECS-1) |
|
Mild steel (MS) |
-570 |
20 |
-180 |
|
Ni2+ modified-Zinc Phosphated mild steel (PMS) |
-460 |
2 |
-20 |
Table 1 Corrosion potentials, current densities (at corrosion potential) and pitting potentials of MS and PMS samples after 8 days of immersion in SC solution10
Calcium addition: Incorporation of calcium ions into zinc-phophate bath has resulted in considerable reduction in the grain size, more compact structure and better corrosion prevention ability.49,50 Jalili12 showed that adding Ca2+ to the phosphate bath led to a significant reduction in corrosion current to a value of about one-fifth to one-eighth when compared to that recorded by the control. This addition also enhanced the bond strength between the rebar and the concrete by about 74%. They explained this enhancement by the mechanical interlocking, which was the dominant mechanism. The zinc phosphate baths modified by calcium ions (Ca2+) contain rhombic crystals of scholzite Zn2Ca (PO4)2.2H2O.28 Zeng21 observed that adding calcium ions into the phosphate bath, the morphology changed from river-bed-like sheets to flower-type structure, which was more compact, crack-free and showed significant enhancement of anticorrosion properties.
Copper addition: Copper cations are basically used for their active role in the germination and growth of phosphate crystals.51,52 Copper also refined the crystal structure and enhanced the coverage of the surface significantly.53 This was attributed to the effect of CU-Fe galvanic couple on dissolution of iron and acceleration of crystal deposition.53 Leoni54 revealed that the addition of copper to the phosphating bath led to a drastic reduction in the corrosion rate. Kulinich & Akhtar48 reported that adding Cu2+ to the zinc phosphate coating bath at concentrations up to 10ppm caused significant changes in coating morphology, adhesion, and corrosion protection. Copper sometimes replaces nickel for environment-related reasons. Although copper is considered to have nearly the same toxicity as nickel, it can be used in much lower concentration in the phospahting bath.32 According to,32 Cu2+ may deposit as copper metal on the surface. Therefore, the metal surface exposed between the crystals is, in fact, a surface of copper rather than the base metal.32
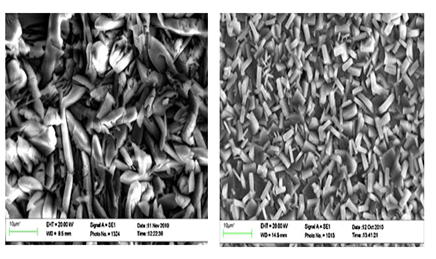
Manganese addition: Manganese (Mn2+) behavior, to some extent, in the phosphating bath is similar to that of Ni2+. The inclusion of Mn2+ into the phosphate bath resulted in more ordered structure and enhanced corrosion resistance properties.55,47 In addition, Mn2+ may replace Zn2+ in the hopeite crystal lattic. According to Su & Lin,40 the major role of Mn2+ in the phosphate treating solution is to enhance the impingement of Mn2+, PO43-, and Zn2+ in the solution and accelerating the formation of Mn2Zn(PO4)2.4H2 Rezaee22 also observed this formation. However, contrary to the results related to the grain refinement effect accompanying with adding Mn2+, Rezaee22 observed a plate-like morphology on the sample treated by the phosphate coating containing Mn2+, Figure 4. This plate-like morphology of the phosphate crystals caused a higher surface coverage than needle like phosphate crystal. They showed that addition of Mn2+ to the phosphate bath solution caused an increase in phosphate crystal size. The competition between Mn2+ and Zn2+ cat ions to form phosphate complexes caused a decrease in the number of zinc phosphate crystals. As a result, the crystal size in the presence of Mn2+ became higher than that formed in the absence of the additive. In addition, adding Mn2+ reduced the phosphate coating porosity and improved surface coverage which led to an enhanced corrosion resistance.22
Molybdenum addition: Molybdenum could be considered one of the promising compounds to be incorporated into the phosphating bath.56 Lin56 reported that addition of Mo2+ resulted in a significant corrosion inhibition capability, almost close to that of chromate conversion coatings. They attributed this enhancement in corrosion resistance to the modified morphology of coating from needle-like to fine flake-shaped structure and to the increase in coating compactness, coating mass and coverage (Figure 4).56
Nano-silica addition: Sheng24 has recently introduced SiO2 nanoparticles in the phosphating bath as a novel accelerator. They demonstrated that with addition of these nano-particles the crystal structure of the phosphate conversion coating was refined, and the coating weight was increased resulting in a much better corrosion prevention capability. In addition, the presence of SiO2 nanoparticles influenced the preferential orientation of the phosphate crystals and the coating morphology Sheng.24 Nano-silica in the phosphating bath acts as a catalyst for the formation of more dense and compact phosphate coating on the rebar surface.11 Addition of nano-silica also ensured about 11% increase in bond strength between concrete and reinforcement.11 In addition, nano-silica modified zinc phosphate coated steel showed the lowest corrosion currents (~0.375µA.cm-2) when compared to nano-silica free coatings (~0.535 µA.cm-2).11 This, according to Manna11 can be explained by the presence of more zinc-bearing phosphate compound in this phosphate coating as zinc-bearing phosphate compounds are less reactive to aggressive chloride solution and better insulating capability against charge transfer than the iron-bearing phosphate compounds.11 The dissolution rate of iron from steel surface is slower for nano-silica containing phosphate solution because nano silica is adsorbed on the steel surface and thereby hinders the dissolution of iron.11 Finally, It is worth mentioning that in the automotive industries, the phosphate conversion coating process is usually performed in a trication bath, which is mainly composed of Zn2+, Ni2+, and Mn2+ ions.57,14 This tri-cations zinc phosphating bath led to a significant refinement of crystals, as shown in Figure 5.14
Sludge formation: Dissolved Fe3+ ions from the pickling reaction are precipated as iron (III) phosphate as in the following reaction:30
The created sludge in the process is normally filtered out from the solution utilizing some sort of filter media or equipment (Figure 5).30 Phosphate sludge is a by-product that could be suitable for ceramics manufacturing. However, because of shortage of alumina and silica, it should be enriched with aluminosilicates such as clays.58 Caponero & Tenorio59 suggested using this sludge in cement clinker. Their results showed no damage to the clinkerization process with an addition of up to 7.0% sludge. In addition, According to Ucaroglu & Talinli,60 the phosphate coating sludge after being treated by solidification/stabilization can be used in a matrix of Portland cement. Baldy61 also studied the possibility of reclaiming this sludge to a zinc-phosphate replenishment solution and other useful products.
This paper was an attempt to briefly review effects of some additives such as alkali metal ions and SiO2 nanoparticles on zinc phosphate coating properties. Addition of certain compounds such as metal ions or nanoparticles to the zinc phosphating bath led to improvements in the zinc coating performance in terms of reinforcement corrosion resistance and the bond strength between concrete and steel. Further studies on the chemical stability of the modified zinc phosphate coatings on reinforcement are highly recommended. Long term tests when these coatings are exposed to chloride ions are also recommended.
None.
Author declares that there is no conflict of interest.

©2017 al-Swaidani. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.