MOJ
eISSN: 2572-8520


Mini Review Volume 2 Issue 6
1Department of Environmental Technology and Management, Kasetsart University, Thailand
2Department of Environmental Engineering and Water Technology, UNESCO-IHE Institute for Water Education, 2601DA Delft, The Netherlands
Correspondence: Suthee Janyasuthi Wong, Department of Environmental Technology and Management, Faculty of Environment, Kasetsart University, Bangkok 10900, Thailand
Received: April 03, 2017 | Published: June 5, 2017
Citation: Janyasuthwiong S, Rene ER (2017) Bioprecipitation - A Promising Technique for Heavy Metal Removal and Recovery from Contaminated Wastewater Streams. MOJ Civil Eng. 2017;2(6):191-193. DOI: 10.15406/mojce.2017.02.00052
With balanced industrial and infrastructural growth and stability in many developing countries, metal reserves are fast depleting and some of the metals are predicted to be exhausted in a few decade. Metal recovery from waste (liquid) streams, for example - acid mine drainage, is one classical option to achieve sustainable resource utilization. Bioprecipitation is a novel approach to recover metals with the help of biologically produced sulfide in suitable bioreactor configurations. Using this technique, stable metal precipitates are formed, which can be subsequently recovered for further industrial use.
Keywords: metals, bioprecipitation, sulfide precipitation, metal recovery, acid mine drainage, technological and societal challenges
Technological advancements, rapid industrialization in developing countries as well as countries in transition, urbanization and global population growth has started to cause an imbalance in the availability of global natural resources. The production rate of metals can no longer be compared to the daily, rapid utilization rate and it is noteworthy to accentuate the fact that many resources have already started to show its deprivation. Presently, the shortage of metals is being felt in many developed or industrialized nations due to over demand and this has resulted in the escalation of global metal market prices. Leading metal mining companies are trapping the unexplored metal reserves in developing countries and selling them at the international market due to the attractive price. As a consequence of this mass mining, exploration and acquisition activity, several metals will be soon exhausted from the earth’s surface.
On the other hand, metal contaminated wastewater especially from anthropogenic source like acid mine drainage (AMD) is a persistent issue, specifically in developing countries and in places where there are abandoned mining sites. Other sources of metal containing waste stream includes industries such as metal finishing, metal plating, semiconductor manufacturing, steel making, electrical power plants, quarry related operations, etc, among others (Figure 1). Due to its tendency to bioaccumulate and biomagnify in the food chain, metal toxicity has caused adverse effects to both living beings and the environment.
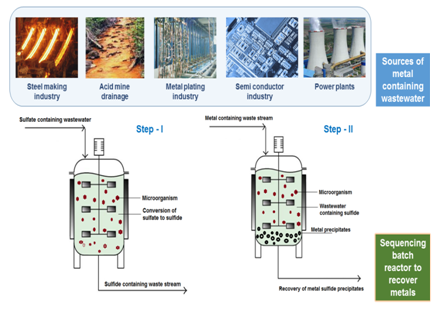
Nowadays, due to the arising knowledge on metal contamination and metal scarcity issues, recovery of metals from the waste stream and reusing the metals has been the focus of several research groups and process industries. Such an effort will not only contribute to sustainability of metal reserves, but it will also avoid further resource depletion. Among the different techniques and technologies proposed in the literature for the removal and recovery of metals from contaminated waste streams, biological treatment, specifically bioprecipitation, is an environmental friendly approach. From a process and mechanism view point, this approach relies on the use of sulfate containing wastewater to produce sulfide through the anaerobic pathway and subsequently the metals are recovered in the form of metal sulfide precipitates.
Bioprecipitation for metal recovery
In general, precipitation is one of the easiest approaches for wastewater treatment wherein the focus or objective of the treatment is to recover valuable by-products from the waste stream. In conventional precipitation, this technique often requires the addition of chemicals to improve the settling ability of the precipitates and therefore, it eventually leads to high operational costs, the idea of using biological processes to facilitate metal precipitation was thought upon for environmental engineering applications. As the name suggests, bioprecipitation uses microorganisms, especially bacteria. During bioprecipitation the produced metabolites react with metals present in the wastewater and forms metal precipitates, i.e. it converts the metals from its aqueous phase into solid phase. According to Kumari et al.,1 microorganisms can facilitate the metal immobilization step via carbonate precipitation. However, the most stable product is metal sulfide over others strategies that use carbonate, phosphate or hydroxide.2 In this domain of research, sulfate reducing bacteria (SRB) is commonly mentioned in many metal bioprecipitation publications due to its metabolic pathways that produces sulfide during the anaerobic treatment of sulfate containing wastewater.
Opinion on technological challenges
Although this technology has proven to be very efficient in several laboratory scale a investigations, there are still some challenges for its application in full or industrial scale systems.3–5
Environmental biotechnology uses biocatalysts for the remediation of a wide variety of pollutants of environmental interest. Bioprecipitation can be applied in both developing and developed countries; however, the cost-benefit of reusing or selling the recovered metals may vary depending on the company’s investment on infrastructure. Besides, the separation and purification steps dictate the costs of secondary metals in the global metal market. Future research in this field should be aimed at performing cost-benefit analysis of the bioprocess, process integration for resource recovery, the development of phenomenological models to describe the bioprecipitation mechanism and investigation on the enzymatic pathways involved.
None.
The author declares no conflict of interest.

©2017 Janyasuthwiong, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.