MOJ
eISSN: 2574-819X


Research Article Volume 2 Issue 2
Department of Pharmaceutical and Biochemical Technology, University of São Paulo, Brazil
Correspondence: Mauri Sergio Alves Palma, Department of Pharmaceutical and Biochemical Technology, University of Sao Paulo, 580, Professor Lineu Prestes Avenue, São Paulo, SP, Brazil, Tel +5511 3091 2387
Received: February 16, 2018 | Published: March 22, 2018
Citation: Consolini G, Junior ENS, Vieira RO. Using capillary microreactor to synthesize three thiazolidine–2, 4–dione derivatives. MOJ Biorg Org Chem. 2018;2(2):83-86 DOI: 10.15406/mojboc.2018.02.00060
The use of flow microreactors in the chemical and pharmaceutical industries offers a number of advantages due to their reduced size compared to batch reactors, the reactors more commonly used by this type of industry. This work aimed at the transposition of the batch process into the continuous flow process in capillary microreactor of the synthesis of (Z)-5-(4-methoxybenzylidene)thiazolidine-2,4-dione (1), (Z)-5-(4-hidroxy-3-methoxybenzylidene)thiazolidine-2,4-dione (2) and (Z)-5-(4-nitrobenzylidene)thiazolidine-2,4-dione (3) which are the base for obtaining drugs through the reaction of the thiazolidine-2,4-dione (TZD) with the aryl aldehydes 4-methoxybenzaldehyde, 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde, respectively. The 4-methoxybenzaldehyde showed higher reactivity and TZD showed the highest conversion (100%) in this reaction, followed by the synthesis of (3), which exhibited 72% TZD conversion. The synthesis of (2) showed the lowest conversion of TZD, 62%, possibly due to steric hindrance.
Keywords: microreactors, sustainable processes, mass transfer, flexible, pyrrolidine, 4-nitrobenzaldehyde, pyrrolidine amount, 4-hydroxy-3-methoxybenzaldehyde, 4-methoxybenzaldehyde, 4-nitrobenzaldehyde, homogenization
The global need for more sustainable processes has reduced the use of natural resources. The search for processes that causes less harm to the environment, combined with better industrial practices, is becoming more and more present.1
In the chemical and pharmaceutical industries, one of the major difficulties for commercialization of a new drug is to produce it on a large scale where there are difficulties of mixing, homogenizing the reagents, heat and mass transfer.2
The challenge of developing new technologies more productive, flexible, and safer with less energy consumption and less waste generation have led to the necessity of process intensification.3 The process intensification has premises as the reduction of the order of magnitude of the industrial plants with the objective of miniaturizing the equipment and integrating the reactions with the stages of separation.4
With these facts presented, microreactors (MRs) appear as a process alternative, since they have advantages such as small distances for diffusion, which guarantees better homogenization, higher conversion than in the usual batch processes, high surface/volume ratio, which provides a better heat transfer,5 lower waste generation that generates less impact to the environment and a lower cost of effluent treatment for the industry.6
The increase in production in batch reactors is generally achieved by increasing the volume of the reaction vessel (scaling-up), which usually brings difficulties in maintaining efficient heat transfer and mixing. This fact does not occur in MRs, since one of the ways to increase the production scale is to add to the system other MRs in parallel (numbering-up).7
In order to show the advantages of MRs for chemical and pharmaceutical industries, the synthesis of important pharmaceutical intermediates was studied. The products are (Z)-5-(4-methoxybenzilidene)thiazolidine-2,4-dione (1), (Z)-5-(4-hydroxy-3-methoxybenzilidene)thiazolidine-2,4-dione (2) e (Z)-5-(4-nitrobenzilidene)thiazolidine-2,4-dione (3), synthesized through the reaction of 2,4-thiazolidinedione (TZD) with 4-methoxybenzaldehyde (a), 4-hydroxy-3-methoxybenzaldehyde (b) and 4-nitrobenzaldehyde (c), respectively, as outlined in Figure 1. TZD has been extensively studied in the medicinal chemistry due to its characteristic of affecting genes and presenting different biological activities such as the action against diabetes mellitus type II, anti-bacterial, anti-inflammatory, anti-oxidant, anti-fungal, anti-convulsive, anti-ischemic and anti-HIV action.8,9
The experimental procedure was adapted from the literature.10 The microreactor system can be pressurized, and because of that it was possible to conduct experiments at temperatures higher than the normal boiling point of the solvent (methanol). To conduct the tests in the microreactor two solutions were prepared:
The optimum pyrrolidine amount for 4-hydroxy-3-methoxybenzaldehyde was 3.2mmol (1.0 eq.), and for 4-methoxybenzaldehyde and 4-nitrobenzaldehyde it was 4 mmol (1.25 eq.). The solutions were well mixed to ensure complete dissolution of the reactants. The heating of the system was accomplished with a silicone oil bath in a 1 L beaker, which was kept on a heating plate with magnetic stirrer.
In the microreactor experiments, the influence of mean residence time was determined by varying the flow rate. Experiments were carried out with mean residence times of 2, 4, 8, 12, 16, and 20 min in each temperature. Table 1 shows the temperature and pressure conditions for the runs in the microreactor. Samples were collected at each mean residence time and analyzed in HPLC-UV, Prominence 20AD model from Shimadzu, equipped with Ascentis C18.5μm, 25cm column for determination of TZD conversion. The reaction was monitored by TLC.
Solvent |
T (°C) |
P (bar) |
Methanol |
65 |
0.1 |
78 |
1.8 |
|
98 |
3.2 |
|
120 |
6.1 |
|
140 |
10.3 |
Table 1 Experimental conditions for the runs in the microreactor
In order to show that the microreactor may be an alternative to the usual batch process, experiments were conducted with three different aldehydes varying the mean residence time and temperature in order to optimize the proposed syntheses. These results are shown in Figures 2‒4.
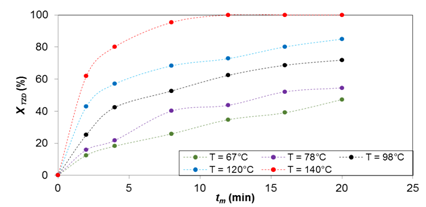
Figure 2 TZD conversion in the reaction of TZD with 4-methoxybenzaldehyde at different temperatures. tm = mean residence time. XTZD = TZD conversion
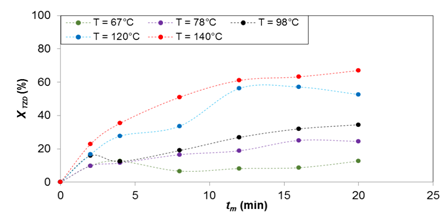
Figure 3 TZD conversions in the reaction of TZD with 4-hydroxy-3-methoxybenzaldehydeo at different temperatures. tm = mean residence time. XTZD = TZD conversion.
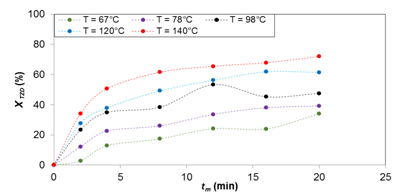
Figure 4 TZD conversion in the reaction of TZD with 4-nitrobenzaldehyde at different temperatures. tm = mean residence time. XTZD = TZD conversion.
Figures 2‒4 show the results for temperatures of 65, 78, 98, 120 and 140 °C for the synthesis of products (1), (2), and (3), respectively. It can be seen that the TZD conversion is proportional to the temperature increase and the mean residence time. The reactant conversion for the synthesis of (1) is 47, 55, 72, 85 and 100%, for product (2) 13, 25, 35, 53 and 67%, and for product (3) 34, 39, 48, 62 and 72%, respectively. All these results are for mean residence time 20 min.
In order to shed light on some TZD conversion results, Figure 5 shows the TZD conversion results for the 3 aldehydes as a function of temperature. The conversion of TZD in the reaction with 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde are approaching with the increase of temperature. At the normal boiling temperature of the solvent, 65 °C, the difference between the conversions was 21%, whereas for 140 °C the difference was only 4%, at nominal values.
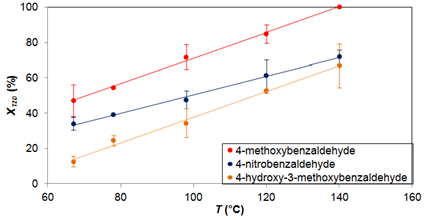
Figure 5 TZD conversion for tm = 20 min for the reaction of TZD with 4-methoxybenzaldehyde, 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde at temperatures ranging from 65 to 140 °C. XTZD = TZD conversion.
The influence of temperature can also be observed by comparing the TZD conversion at the normal boiling temperature of the solvent with the conversion of TZD at the highest temperature studied, 140 °C. For the synthesis with 4-methoxybenzaldehyde, the increase in TZD conversion was 53%; for the synthesis with 4-hydroxy-3-methoxybenzaldehyde the increase was 54% and for the synthesis with 4-nitrobenzaldehyde the increase was 37%, in nominal values. These results show that the synthesis with 4-hydroxy-3-methoxybenzaldehyde is more influenced with the increase in temperature.
To determine the order of the reaction, batch tests in methanol, using pyrrolidine as promoter base, with reactants in excess were carried out. In this method an excess of one of the reactants is used, enough that its concentration as a function of time is practically constant when compared to the concentration of the other reactant. In this way, the rate equation is treated mathematically according to the model of pseudo-first order, Eq. 1, described in HOUSE (2007).11
Tests with excess of TZD (not shown here) were also performed to determine the reaction order for each of the three aldehydes. It was also verified that the reaction in relation to aldehydes is first order. The adjustment of the global second order model (order 1 to each reagent) with stoichiometric feed of the reagents, shown in Eq. 2, was also tested (HILL and ROOT, 2014). Eq. 1 was adjusted to the TZD concentration results as a function of time (excess of aldehyde). These results are shown in Figure 6.
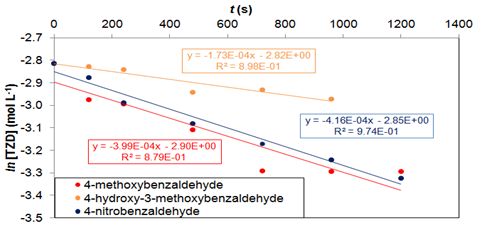
Figure 6 Graph of ln [TZD] versus time for the reaction of TZD with 4-methoxybenzaldehyde, 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde in order to determine the reaction order. T=67 °C. Batch tests.
Figure 6 show that there was a good adjustment of the pseudo-first order model to the experimental results for the three products in the batch assays at 67 °C. The linear correlation coefficients for 4-methoxybenzaldehyde, 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde were 0.879, 0.898 and 0.974, respectively. Tests with excess of TZD (not shown here) were also performed to determine the reaction order for each of the three aldehydes. It was also verified that the reaction in relation to aldehydes is first order. The adjustment of the global second order model (order 1 to each reagent) with stoichiometric feed of the reagents, shown in Eq. 2, was also tested (HILL and ROOT, 2014). Eq. 2 was adjusted to the TZD and aldehyde concentration as a function of time. These results are shown in Figure 7.
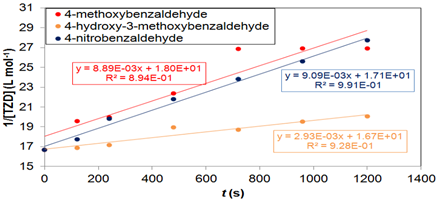
Figure 7 Graph of 1/[TZD] versus time for the reaction of TZD with 4-methoxybenzaldehyde, 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde in order to determine the reaction order. T=67 °C. Batch tests.
Figure 7 show that there was a good adjustment of the global second order model to the experimental results for the three products in batch tests at 67 °C. The linear correlation coefficients for 4-methoxybenzaldehyde, 4-hydroxy-3-methoxybenzaldehyde and 4-nitrobenzaldehyde were 0.894, 0.928 and 0.991, respectively. With the TZD conversion results in the microreactor, it was possible to determine the second order reaction rate constant (k) of the studied reactions and the activation energy (Ea). The values of k were determined by the kinetic model of 2nd order presented in HILL and ROOT (2014).12 These results are shown in Table 2.
k (L mol-1s-1) |
Ea (kJ mol-1) |
|||||
|---|---|---|---|---|---|---|
65 °C |
78 °C |
98 °C |
120 °C |
140 °C |
|
|
4-methoxybenzaldehyde |
1.48 x 10-2 |
1.43 x 10-2 |
3.90 x 10-2 |
4.44 x 10-2 |
21.97 x 10-2 |
8.18 |
4-hydroxy-3-methoxybenzaldehyde |
0.11 x 10-2 |
0.32 x 10-2 |
0.80 x 10-2 |
1.66 x 10-2 |
4.91 x 10-2 |
56.11 |
4-nitrobenzaldehyde |
0.47 x 10-2 |
0.81 x 10-2 |
1.43 x 10-2 |
3.11 x 10-2 |
4.10 x 10-2 |
26.02 |
Table 2 Study animal information: ID, gender, age, and dates of data collection for each study animal
The values of Ea, calculated by the adjustment of the Arrhenius model, were, respectively, 8.18, 26.0 and 56.1 kJ mol-1 for the reaction with 4-methoxybenzaldehyde, 4-nitrobenzaldehyde and 4-hydroxy-3-methoxybenzaldehyde, respectively. These results and that shown in (Figure 4) (Figure 5) confirm the highest TZD conversions with 4-methoxybenzaldehyde, since, for this reaction, the activation energy is minimum, indicating that the reaction needs less energy to start. The reaction with 4-hydroxy-3-methoxybenzaldehyde showed the lowest TZD conversion and the highest activation energy. Considering the results discussed above, the advantage of the microreactor is evident, which allows to work with high temperatures, promoting the increase of the productivity of the process with safety, being this one of the main advantages of the microreactor technology.
Under the conditions studied, the best results were obtained for temperature of 140 °C. As the temperature increases, the molecular interaction, reaction rates and, consequently, the reactants conversion will increase. The TZD conversion was higher for 4-methoxybenzaldehyde, with a value of 100% for mean residence time of 20 min, followed by 4-nitrobenzaldehyde and 4-hydroxy-3-methoxybenzaldehyde. The highest influence of temperature was observed for 4-hydroxy-3-methoxybenzaldehyde.
The possibility of pressurizing the system and, therefore, use of temperatures above the normal boiling of the solvents, is one of the main advantages of the application of microreactor technology when compared to the batch reactor. This condition allows the process to achieve higher conversions at lower reaction times. These results confirm the feasibility of the use of micro reactors and their advantages, being a viable alternative for fine chemical industry.
The authors thank FAPESP, Capes and CNPq for financial support.
The authors declare that there is no conflict of interest regarding the publication of this article.

©2018 Consolini, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.