MOJ
eISSN: 2574-819X


Research Article Volume 2 Issue 4
Department of Chemistry, Pondicherry University, India
Correspondence: Vasuki Gnanasambandam, Department of Chemistry, University of Pondicherry- R.V. Nagar, Kalapet, Puducherry-605014, India, Tel +9194 8666 3962, +9194 8666 4962
Received: July 31, 2018 | Published: August 8, 2018
Citation: Gnanasambandam V, Nithya N. Design and synthesis of Azolidinedione/Thiazolidinediones teth-ered benzo[f]chromene derivatives and there in silico evaluation as tubulin inhibitors. MOJ Biorg Org Chem. 2018;2(4):196-199. DOI: 10.15406/mojboc.2018.02.00081
A series of naphthalene based hybrid heterocyclics were designed and synthesized by the replacement of benzene ring with napthalene on 4-substituted 4H-chromenes. As a part of our continuous efforts in ac-cessing the bioactive compounds by using simple techniques, we report the synthesis of azolidinedione/thiazolidinediones tethered 4-substituted benzo[f]chromenes derivatives under greener reaction conditions and in silico evaluation of anticancer activity. Docking studies showed that the synthesized compounds exhibit good predicted binding affinities at the colchicine binding site of tubulin (Figure 1).
Keywords: 4-Substituted-4H-Chromenes, hybrid heterocyclics, azolidinediones, thiazolidinediones, col-chicine binding site
The small molecules that target microtubules is an attractive and an active area in cancer drug discovery.1‒4 These molecules bind to the tubulin, an α, β- heterodimer and disrupt the dynamics of microtubule. Microtubule targeting agents can be classified into two categories. Microtubule stabilizing agents such as paclitaxel, docetaxel, epothilones, and discodermolide binds to the tubulin polymer and stabilize the microtubules. Microtubule destabilizing agents such as vinca alkaloids, colchicine and combretastatins binds to tubulin dimers and cause destabilization.5 The equilibrium between tubulin and microtubule is altered, which results in disruption of mitotic spindle. This effects a critical transition in the cell cycle, leading to cell death. Out of these different pockets on tubulin, colchicine is a significant source of inspiration for the design of new drugs as the colchicine binding site inhibitors binds with high affinity at the interface of α and β-tubulin. These are effective against multidrug mechanisms but however, the potential clinical applications of colchicine site tubulin inhibitors have been nullified by the significant toxicities against the normal cells, low solubility, and low bioavailability.6,7 The increase in resistance of cancer cells against current clinical drugs and poor tolerance of the existing anticancer drugs, intensifies the need to identify new molecules as anticancer drugs with high potency, minimal side effects.
In an endeavor to find the potential chemotherapeutic agents, a substantial effort has been devoted on the design and development of heterocycles embedded small organic molecules. The thiazolidinedione ring has been used as scaffold to develop novel class of anticancer agents with a wide range of cytotoxicity against many human cancer cells.8 From the literature,9,10 it was observed that numerous compounds containing thiazolidine 2,4-dione and rhodanine have been reported as potential anticancer agents. Also several chromene derivatives were reported as effective anticancer agents; particularly 2-Amino-3-cyano-4-aryl-4H-chromene derivatives are promising apoptosis inducing agents. The presence of the 4-aryl moiety, the 3-cyano group, and the 2-amino functionality were essential features for the anticancer activity of these chromene analogues.11 Patil and co-workers12 have reported that naphthalene tethered 4-substituted-4H chromene (Figure 2) was most active against all the cell lines and showed most potent tubulin polymerization inhibitory activity. Inspired by these results, we hereby report the design and synthesis of azolidinedione/thiazolidinediones tethered benzochromene analogues and their insilico evaluation as tubulin inhibitors.
We are actively engaged in accessing hybrid heterocyclic scaffolds through multicomponent reactions by integrating the concepts of diversity oriented synthesis and bioisosterism particularly build/couple/pair strategy.13‒15 Recently we have reported the synthesis of double ring replacement analogues of 4-heterocycle substituted 4H-chromenes.16 The synthesis of azolidinedione/thiazolidinediones tethered benzochromene analogues were carried out as outlined in Figure 3 & Figure 4. We have initiated our investigation by a model three component reaction between 1-formyl-2-hydroxy-napthalene (1), hydantoin (2) and malononitrile (3) in water, in the presence of piperidine, as a base. The reaction yielded benzo[f]chromene derivatives in 30 min with 94% yield. Later the reaction was optimized using different solvents and bases. It was observed that the best results were obtained when the reaction was conducted in water and piperidine as a base for 30min at room temperature. The product was confirmed by 1H, 13C NMR and HRMS spectra. A set of compounds (4a-d) were synthesized with different Heterocycle carbon nucleophiles (2a-d).The results were summarized in Table 1.
Docking studies
After establishing a three component reaction to afford 4-heterocycle-4H benzo[f]chromenes, We have per-formed a molecular docking to investigate the possible interactions and binding conformation for this set of compounds at the colchicine binding site of tubulin, which may give a suggestion about their proposed mechanism of action. All the stereoisomer’s of the synthesized compounds were docked at the colchicine binding site of tubulin (PDB entry: 1SAO) using auto dock vina,17 and the docking results were summarized (Table 1, entries 1-16). It was observed that R,S configuration of 4a was found to exhibit maximum predicted binding affinity and almost all the stereoisomer’s have similar binding affinity. These values are comparable to colchicine, EPC2407 and Podophyllotoxin. All the synthesized ben-zochromene analogues bind at and near the colchicines binding site of tubulin. UCSF Chimera visualisation software was used.18 The overlap of RS isomer of 4a with the colchicine at the binding site of tubulin is displayed (Figure 5).
The 2-d images were processed using Ligplotplus.19 A critical analysis of 4a-R, S-tubulin complex (Figure 6) revealed the key interactions that bind 4a-R,S with colchicine binding site of tubulin. NH group on the azolidinedione ring was located within hydrogen-bond distance of (3.11A°) with Thr α179. Oxygen atom of azolidinedione was located within hydrogen-bond distance of (2.80) with Asn α101. The ben-zochromene part of 4a-R,S was embedded in a pocket bounded by Lys β254, Ala β250, Leu β255 Leu β248 Lys β352 and while azolidine ring of 4a-R,S interacts with the Asn α101side chain and Thr α179 via attractive contacts through heteroatom’s (Figure 6). Finally, we can conclude that the synthesized 4-substituted benzo[f]chromenes exhibit similar binding interactions with colchicine binding site similar to 4- substituted chromenes. This suggests that these set of compounds may exhibit significant tubulin inhibitory activity, and may contribute to anti-cancer activity.
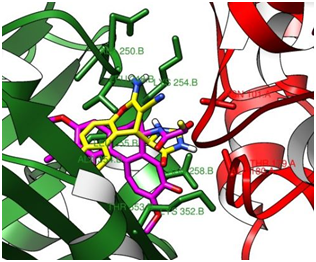
Figure 5A view of the binding conformation of 4a-R, S (yellow) overlapping with colchicine (pink) at the colchicine binding site of tubulin. Tubulin is displayed as a flat ribbon with α-tubulin coloured red and β-tubulin coloured green.
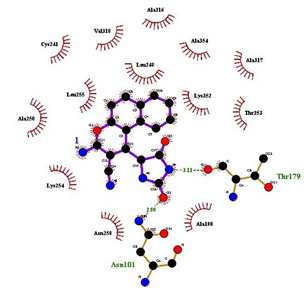
Figure 6 2-d representation of hydrogen bonding and hydrophobic interactions of 4a-R, S with colchicine binding site of tubulin.
S No. |
Compound |
Binding affinity |
S No. |
Compound |
Binding affinity (Kcal/mol) |
1 |
4a-R,S |
-9.9 |
9 |
4c-R,S |
-9 |
2 |
4a-S,R |
-9.7 |
10 |
4c-S,R |
-9 |
3 |
4a-R,R |
-9.7 |
11 |
4c-R,R |
-9.4 |
4 |
4a-S,S |
-9.5 |
12 |
4c-S,S |
-8.9 |
5 |
4b-R,S |
-9.5 |
13 |
4d-R,S |
-9.5 |
6 |
4b-S,R |
-9.5 |
14 |
4d-S,R |
-9.3 |
7 |
4b-R,R |
-9.3 |
15 |
4d-R,R |
-9.5 |
8 |
4b-S,S |
-9.5 |
16 |
4d-S,S |
-9.5 |
Table 1 Predicted binding affinities of synthesized compounds at the colchicine binding site of tubulin
Note: R=R configuration; S= S configuration.
Chemistry and materials
Commercial reagents were used without further purification. Melting points were recorded on a Veego capillary melting point apparatus and were uncorrected. For compounds 1H NMR (400MHz, DMSO-d6) and 13C NMR (100MHz, DMSO-d6) spectra were recorded in DMSO-d6 on a Bruker 400MHz spectrometer using tetramethylsilane (TMS, δ=0) as an internal standard at room temperature. Mass spectra were recorded on Agilent 1200 LC/MS-6110 mass spectrometer.
General procedure for synthesis of 3-amino-1-(2,5-dioxoimidazolidin-4-yl)-1H benzo[f]chromene-2-carbonitrile 4a
A mixture of 1-formyl-2-hydroxynapthalene (1mmol), hydantoin (1mmol) and malononitrile (1mmol) were taken in water (5ml) in a round bottom flask and the reaction mixture was allowed to stir at room temperature until the starting materials disappear on tlc. The yellow colour pre-cipitate was filtered and washed several times with water (20mL) and cold ethanol (5mL) and then dried. The product 4a obtained as light brown solid.
3-amino-1-(2,5-dioxoimidazolidin-4-yl)-1H-benzo[f]chromene-2-carbonitrile 4a
Yield (94 %); mp 258-263°C; 1H NMR (400MHz, DMSO-d6) δ 9.89 (s, NH), 8.60 (s, NH), 7.9-7.99 (m, 3H), 7.64-7.78 (m, 1H), 7.49-7.54 (m, 1H), 7.22 (d, J=8.8 Hz, 1H), 7.10 (s, NH2), 4.66 (d, J=4Hz, 1H), 4.38 (d, J=4Hz, 1H) ppm; δ 13C NMR (100MHz, DMSO-d6) δ, 178.40, 174.29, 163.39, 148.20, 130.75, 129.49,129.07, 128.81, 127.55, 125.07, 121.83, 117.16, 112.54, 103.11, 65.20, 47.22, 35.13ppm. HRMS (ESI) Calcd for C17H12N4O3 [M+Na] 320.09amu, found 320.10amu.
3-amino-1-(5-oxo-2-thioxoimidazolidin-4-yl)-1H-benzo[f]chromene-2-carbonitrile 4b
Yield (89%); mp 252-255°C; 1H NMR (400MHz, DMSO-d6) δ 11.69 (s, NH), 9.80 (s, NH), 7.91-7.99 (m, 4H), 7.49-7.54 (m, 2H), 7.12 (s, NH2) 4.66 (d, J=4Hz, 1H), 4.38 (d, J=4Hz, 1H) ppm; δ 13C NMR (100MHz, DMSO-d6) δ 183.7, 174.29, 163.39, 148.20, 130.75, 129.49, 128.81, 127.55, 125.97, 125.02, 122.08, 121.83, 119.98, 112.54, 65.20, 47.22, 35.13. ppm. HRMS (ESI) Calcd for C17H12N4O2S [M+Na] 336.07amu, found 336.08amu.
3-amino-1-(4-oxo-2-thioxothiazolidin-5-yl)-1H-benzo[f]chromene-2-carbonitrile 4c
Yield (91%); mp 256-260°C; 1H NMR (400MHz, DMSO-d6) δ 7.8-8.3 (m, 3H), 7.89 (d, J=8.4Hz, 1H), 7.6-7.70 (m, 1H), 7.5-7.54 (m, 1H), 7.21 (d, J=8.8 Hz, 1H), 7.05 (s, NH2), 4.94 (d, J=2.4 Hz, 1H), 4.48 (d, J=2Hz, 1H)ppm; δ 13C NMR (100MHz, DMSO-d6) δ 173.16, 164.15, 158.16, 145.97, 145.53, 144.03, 131.22, 127.84, 119.87, 62.77, 58.12, 46.70, 34.83, 18.65ppm. HRMS (ESI) Calcd for C17H1N3O2S2 [M+Na] 353.03amu, found 353.03amu.
3-amino-1-(2,4-dioxothiazolidin-5-yl)-1H-benzo[f]chromene-2-carbonitrile 4d
Yield (86%); mp 260-265 °C; 1H NMR (400MHz, DMSO-d6) δ 11.85 (s, NH), 7.91-7.98 (m, 4H), 7.5-7.58 (m, 2H), 7.12 (s, NH2), 4.66 (d, J=4Hz, 1H), 4.38 (d, J=4Hz, 1H) ppm; δ 13C NMR (100 MHz, DMSO-d6) δ 181.70, 170.29, 163.43, 147.20, 130.75, 129.37, 129.0, 128.95, 127.83, 125.22, 121.85, 120.19, 116.78, 115.82, 66.19, 49.34, 35.40ppm. HRMS (ESI) Calcd for C17H12N4O3 [M+Na] 320.09amu, found 320.10amu.
In conclusion, we have designed and synthesized azolidinedione/thiazolidinediones tethered ben-zochromene analogues under greener reaction conditions. Docking results show that all the four synthesized compounds bind at colchicine binding site of tubulin and exhibit good predicted binding affinities. In vitro studies of the synthesized compounds are in progress and will be reported in future publications.
Author declares that there is no conflict of interest.

©2018 Gnanasambandam, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.