MOJ
eISSN: 2574-819X


Review Article Volume 2 Issue 2
1University of Massachusetts Medical School, USA
2Department of Science and Humanity (Chemistry), Karunya Institute of Technology and Sciences, India
3Indian Institute of Technology Madras, Chennai, India
Correspondence: Sivaraman Balasubramaniam, Senior Research Scientist, Indian Institute of Technology Madras, Chennai, India, Tel +9177 1880 5113
Received: March 07, 2018 | Published: March 29, 2018
Citation: Sankaran GS, Arumugan S, Balasubramaniam S. Application of chiral sulfoxides in asymmetric synthesis. MOJ Biorg Org Chem. 2018;2(2):93-101. DOI: 10.15406/mojboc.2018.02.00062
Chiral sulfoxides are used as a toolbox for the synthesis of enantiomeric/diastereomeric compounds, which are used as precursors for the pharmaceutically/chemically important molecules. The current review focuses on applying these chiral sulfoxides towards the synthesis of the compounds having stereogenic center. In general, the stereogenic center induced by the sulfoxide is able to direct the stereochemistry of further transformation necessary to complete the total synthesis of bioactive molecules. The nature of the reactive conformation of the sulfoxide is strongly dependent on the nature of the substituents at C-α and/or C-β.
Over the last three decades, the sulfinyl group has received considerable attention in asymmetric synthesis1‒5 as a chiral tool. The sulfinyl group is widely used as to bring about numerous asymmetric transformations. The effectiveness of the sulfoxide in diastereoselective auxiliary-induced reactions is mainly due to the steric and stereo electronic differences existing between the substituents on the stereogenic sulfur atom: a lone electron pair, oxygen, and two different carbon ligands, which are able to differentiate the diastereotopic faces of a proximal or even remote reaction center. Besides the high configurational stability of the sulfinyl group,6,7 the existence of several efficient methods to obtain homochiral sulfoxide as well as their synthetic versatility has led to a substantial growth of the use of these chiral starting materials in the synthesis of enantiomerically enriched compounds and in the total synthesis of numerous biologically active natural products.
Asymmetric oxidation of the thioethers
A variety of methods are available to obtain optically active sulfoxides, these include optical resolution, asymmetric oxidation and asymmetric synthesis. The first example of asymmetric oxidation of sulfides to sulfoxides were independently reported by Pitchen et al.8 and Di Furia et al.9 using a modified Sharp less epoxidation reagent [Ti(OiPr)4/(+)-DET/tBuOOH]. Further development of this methedology10,11 showed an increase in the optical purity of the resulting sulfoxide by replacing the tert-butylhydroperoxide with cumenehydroperoxide.
The use of (R)-(+)-binaphthol12 instead of DET (Figure 1) as chiral ligand improved the modest ee (60-70%) achieved in the transformation of some methyl aryl sulfides into sulfoxides to 96% ee by taking advantage of the kinetic resolution process which occurred in a further oxidation step of one of the sulfinyl enantiomer to sulfone.13 The titanium-binaphthol complex catalyzes not only the asymmetric oxidation but also the kinetic resolution process.
More general are the application of the stoichiometric chiral oxidizing reagents described by Davis et al.14,15 The enantiopure (camphorsulfonyl)oxaziridines and their 8,8-dichloro derivatives available in both antipodal form, afforded sulfoxides with ee up to 96%.
Dargo et al.16 came up with a catalytic approach to enantiomerically pure alkyl aryl sulfoxides using a combination of 3,5-diiodo Schiff base ligands and [VO(acac)2] as the catalyst in the presence of H2O2 as oxidant (Figure 2). This combination proved to be effective affording sulfoxides possessing high ee and in high yields. Both enantiomers of the ligand can be easily prepared on multi gram scale and can be stored for prolonged periods without any loss of optical purity.
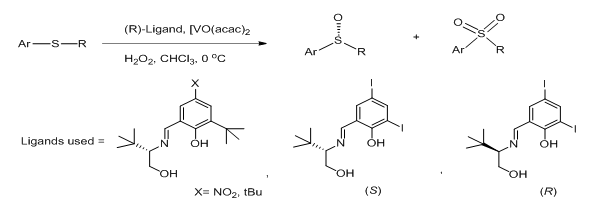
Figure 2 Catalytic approach to enantiomerically pure alkyl aryl sulfoxides using a combination of 3,5-diiodo Schiff.
Nucleophilic substitution on chiral sulfur derivatives
The most widely used approach to enantiomerically pure sulfoxides is the Anderson synthesis17,18 based on the nucleophilic substitution on diastereomerically pure (S S)-menthyl p-toluenesulfinate19 with Grignard reagents which occurs with full inversion of configuration at sulfur (Figure 3). The classical method has been extensively used to prepare p-tolyl alkyl or aryl sulfoxides. The usefulness of this method is mainly due to the accessibility of the sulfinylating agent, obtained as mixture of sulfur epimers through esterification of p-toluenesulfinyl chloride. This originally described procedure to obtain enantiomerically pure sulfoxide was further improved and scaled up by equilibrating the epimeric sufinate in the presence of HCl to displace the equilibrium by precipitation of the (S,S) diastereomer in acetone being thus isolating it in 80% yield.
As a chiral auxiliary in the diastereoselective reduction of β-ketosulfoxides
Acyclic β-keto sulfoxides are readily available by the reaction of α-sulfinyl anion with an ester.20,21 The main contribution to the synthesis of optically active secondary carbinols from β-keto sulfoxide is due to Carreno et al.22 The Stereochemical outcome in the reduction of the β-keto sulfoxide can be controlled by the configuration of the sulfoxide, the reducing agent and the absence or presence of a Lewis acid. The reduction of β-keto sulfoxide (RS) 1 with DIBAL gave (RS,S)-carbinol 2 via intramolecular hydride transfer through a six-membered cyclic transition state (I), while the reduction with LiAlH4 or DIBAL in the presence of ZnCl2 as a Lewis acid give the epimer at the hydroxy center (RS, R)-carbinol 3 which is rationalized by a conformationally rigid six-membered cyclic transition state (II) involving chelation of the Lewis acid to the sulfinyl and carbonyl oxygen (Figure 4). This methodology has been employed in enantioselective synthesis of methyl carbinol, 1,2-diols and epoxides which are used as the key intermediates in the total synthesis of natural products.
Methyl carbinol: One of the great advantages of sulfoxides is allowing the asymmetric induction step to be carried out at the very last part of the synthesis via this stereoselective β-keto sulfoxide reduction. A convergent synthesis of dimethyl ether (S)-6,23 a precursor of zearalenone, a naturally occurring macrolide with anabolic and uterotropic properties has been described (Figure 5). The enantiomerically pure hydroxy sulfoxide 4 derived from glutaric anhydride was transformed to the silyl ether of methyl carbinol 5 via silyl ether protection and subsequent desulfurization, which was further elaborated to the natural product.
The anomeric effect was used to advantage in the highly stereoselective cyclization of dihydroxy disulfoxide 8, accessible with both the S and R configuration at both the hydroxylic centers depending on the reduction method utilized in its obtention from the diketone 7. Upon treatment in acidic medium, compound 8 evolved into a spiroketal derivative whose desulfurization afforded compound 9, present in the rectal glandular secretion of certain species of fruit flies (Figure 6).24 The enantiomer could be synthesized from the dihydroxy disulfoxide resulting from reduction with DIBAL/ZnCl2.
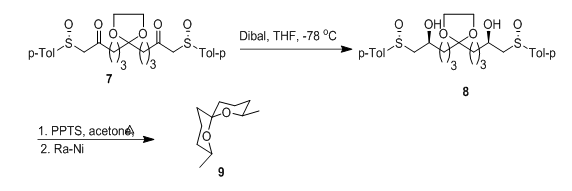
Figure 6 Enantiomer could be synthesized from the dihydroxy disulfoxide resulting from reduction with DIBAL/ZnCl2.
1,2-Diols: The protocol transforming β-hydroxy sulfoxides into terminal 1,2-diols via the Pummerer rearrangement followed by desulfurization or LAH reduction of the resulting hemi-thioketal, has been utilized in the synthesis of several polyhydroxylated natural products. As a showcase, enantiomerically pure β-hydroxy unsaturated sulfoxide 10 was transformed to the triol derivative 11 by dihydroxylation. Compound 11 was further converted to L-penta-O-acetylarabinitol 12 by applying the above strategy (Figure 7).25,26
Epoxides: β-Hydroxy sulfoxides can also be easily transformed into chiral epoxides giving access to a variety of natural products. The asymmetric synthesis of juvenile hormone II 13 was based on the diastereoselective alkylation and carbonyl reduction of β-keto sulfoxide 14.27 Alkylation of 14 produced a (9:1) mixture of (R, Rs) and (S, Rs) whose diastereoselective reduction afforded β- hydroxy sulfoxide 15 which can be transformed to the target by routine synthetic procedures, (Figure 8).
Conjugate additions to α,β-unsaturated sulfoxides
C-C bond formation: Sulfinyl butenolide (S)-17 was used as a Michael acceptor in the synthesis of optically pure 3-substituted butyrolactones. Zinc-bromide promoted benzylic Grignard reagent addition to (S)-17 led, after desulfurization and enolate acylation, to the anticancer agent (-)-podorhizon (18) (Figure 9).28
C-N bond formation: The total synthesis of (R)-carnegine 21 reported by Pyne and coworkers29 was based on the formation of the tetrahydroisoquinoline ring 20 upon cyclisation of (Z)-vinyl sulfoxide 19 under basic conditions. Desulfurization of 20 gave the alkaloid 21 (Figure 10).
Reactions of sulfinyl carbanions
1,2-Addition to carbonyl: Although the addition of simple a-sulfinyl carbanions to carbonyl compounds proceeds with poor diastereoselectivity, the reaction was used as the key step in the synthesis of (+)-disparlure30 25 the pheromone produced by female gypsy moth (Porthetria dispor).
After chromatographic separation of the mixture of hydroxyl sulfoxides resulting from the reaction of lithium anion derived from optically pure sulfoxide 22 and undecanal, disparlure 25, was obtained from the major diastereomer through a sequence involving sulfoxide reduction and base promoted cyclization of the sulfonium salt derived from the thioether (Figure 11).
Additions to α, β–unsaturated compounds: The diastereoselective addition of simple α-sulfinyl carbanions to α, β-unsaturated compounds was reported by Scolastico who described the obtention of prostaglandin intermediate. The conjugate addition of the anion derived from (S)-dithioketal S-oxide 26 to 2-substituted cyclopentenone 27 in the presence of HMPT gave a 52:48 mixture of C-α epimers that were converted into the enantiomerically pure protected aldehyde 28 by desulfurization. The free aldehyde has been used as starting material in prostanoids synthesis (Figure 12).31
The regio- and Stereochemical course of the reaction between ambident sulfinyl allyl anions and cyclic enones has been deeply studied by Binns et al.32‒34 while its application to asymmetric synthesis of natural products was mainly due to Hua et al.35,36 Total synthesis of (+)-hirsutene 32 employed two crucial reactions i.e.
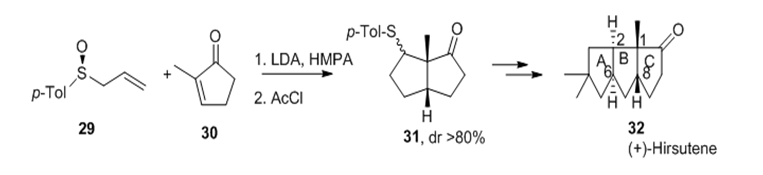
Figure 13 Regio- and stereochemical course of the reaction between ambident sulfinyl allyl anions and cyclic enones
Addition to C=N of imines and sulfinyl imines: The nucleophilic addition of enantiomerically pure α-sulfinyl carbanions to the diasterotopic C=N double bond of imines allows the asymmetric synthesis of β-amino-sulfoxides. Bravo et al. was the first to establish that the addition of chiral sulfoxide-stabilized carbanion to N-(p-methoxyphenyl)aldimines 34 bearing trifluromethyl, pentafluoroethyl and ω-hydrotetrafluoroethyl groups afforded the corresponding enantiomerically enriched amine38 35 with excellent diastereocontrol (Figure 14).
The Stereochemical outcomes of these reactions were rationalized assuming a kinetic control and an enantiodirecting effect of the fluoroalkyl group. The reaction has been extended to N-(PMP)-arylimines, a practical way to prepare enantiopure α-arylglycinols.39
The behavior of enantiomerically pure sulfinylimines with chiral α-sulfinyl carbanions has been reported by Garcia Ruano et al.40 The highest Stereoselectivity is achieved when the configuration at the sulfur atoms of the two reagents are opposite (matched pair), affording only one diastereoisomer (Figure 15). For each pair, the major diastereoisomer has identical configuration at the new stereogenic carbon (R in both case), despite the opposite configuration of the sulfoxide used as nucleophile in each case. This suggests that the stereoselectivity of the reaction is primarily controlled by the sulfur configuration of the starting electrophilic sulfinamide 36.
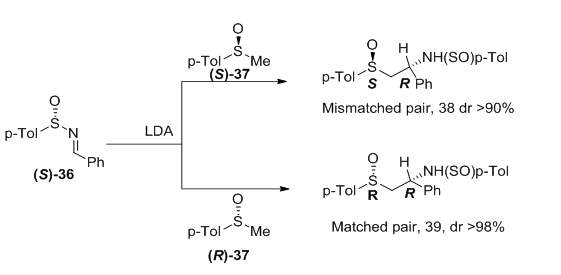
Figure 15 The Stereoselectivity of the reaction is primarily controlled by the sulfur configuration of the starting electrophilic sulfinamide 36.
Pummerer rearrangement
The reaction discovered by Pummerer,41 which brings about the transformation of sulfoxides bearing α-hydrogen into α-acyloxy sulfides upon treatment with acid anhydrides, has been widely used in synthesis (Figure 16). From the stereochemical point of view, this is a self-immolative asymmetric process where the chirality at sulfur is transferred to the α-carbon. Unfortunately, the extent of asymmetric induction reported for the classical Pummerer rearrangement by different authors never exceeded 30% ee.
Pummerer-type conditions have been used successfully for the stereoselective synthesis of β-lactam 43, a precursor of some penicillin antibiotics. Trimethylsilyl triflate promoted Pumerer reaction of sulfoxide 42 afforded the lactam 43 (Figure 17).42
Pummerer-type conditions have been used successfully for the stereoselective synthesis of cyclic compounds by an intramolecular attack of a nucleophile on the sulfenium intermediate formed under the reaction conditions. The use of additive Pummerer-type rearrangement on cyclopentenone 44 allowed a short synthesis of methyl jasmonate 46,43 a perfume essence (Figure 18). The dichlorolactone 45 was transformed to the natural product but with moderate Stereoselectivity (20% ee). A formal synthesis of (-)-serriconine 51 the sex pheromone of the cigarette beetle was recently reported from butyrolactone 48.44
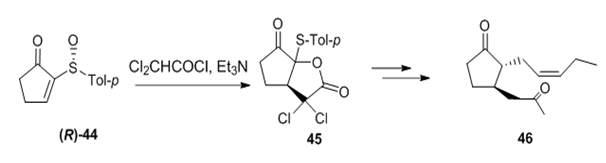
Figure 18 Dichlorolactone 45 was transformed to the natural product but with moderate Stereoselectivity.
The starting vinyl sulfoxide 47 reacted with dichloroketene and after aluminium amalgam and raney nickel treatment, 48 was obtained as a single diastereomer and enantiomer. The intermediate δ-lactone 50 which had been already transformed into the natural product was synthesized after reduction of 48 and homologation of the diol followed by hydrolysis and lactonization (Figure 19).
Bravo et al. reported an unusual Pummerer rearrangement of γ-trifluoro-β-aminosulfoxides.45

Figure 19 Intermediate δ-lactone 50 which had been already transformed into the natural product was synthesized after reduction of 48 and homologation of the diol followed by hydrolysis and lactonizat I.

Figure 20 “Abnormal” rearrangement product was successfully converted to the corresponding β-fluoro-α-aminoalcohol.
Reaction involving vinyl sulfoxides
Cycloaddition reactions: The combination of a Diels-Alder represents a very powerful method for C-C bond formation in a stereocontrolled manner. The sulfinyl group has equally become one of the most interesting chiral inductors in asymmetric Diels-Alder reactions, due to
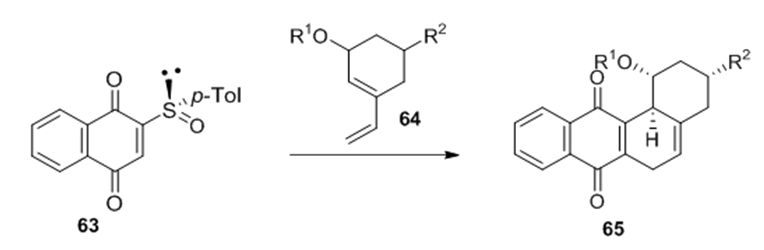
Figure 22 The sulfinyl group proved to achieve a double induction in the Diels-Alder reaction which led to an efficient kinetic resolution of the racemic diene.
These compounds were used in a Diels-Alder reaction with N-phenylmaleimide 67 and phenyltriazolinedione 68 and were found to react with high facial selectivity. Interestingly, the sulfinyl group managed to override the intrinsic allylic stereocontrol of the substrates (Figure 23).50
Complete regioselectivity and Stereoselectivity were observed for the first asymmetric hetero Deils-Alder reaction of 1-sulfinyl dienes with acylnitroso derivatives such as benzyl nitrosoformate. The stereochemical course of the reaction could be explained by considering that the heterodienophile approached the less hindered face of diene that which supported the lone electron pair at sulfur, with the sulfinyl group in an s-trans arrangement with respect to C1=C2 (Figure 24).
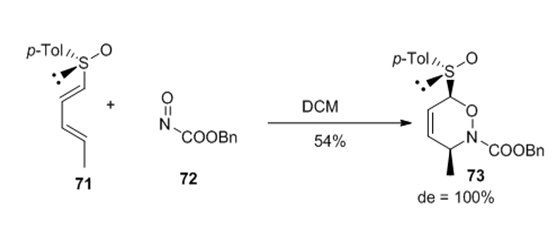
Figure 24 The stereochemical course of the reaction could be explained by considering that the heterodienophile approached the less hindered face of diene that which supported the lone electron pair at sulfur, with the sulfinyl group in an s-trans arrangement with respect to C1=C2.
The asymmetric Diels-Alder reaction of 2-sulfinyl dienes has been used in the total synthesis of (-)-(1S, 5R)-Karahana ether 7451 (Figure 20). The key step involves the cycloaddition of maleic anhydride 76 with 2-sulfinylpentadiene 75 which proceeds in good yield to afford a 4:1 mixture of diastereomeric cycloadducts, which was elaborated to the monoterpenoid (Figure 25).
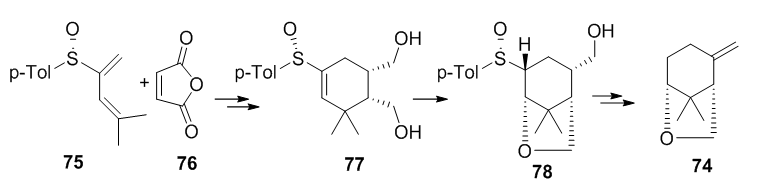
Figure 25 The key step involves the cycloaddition of maleic anhydride 76 with 2-sulfinylpentadiene 75 which proceeds in good yield to afford a 4:1 mixture of diastereomeric cycloadducts, which was elaborated to the monoterpenoid.
Similarly, hetero-Diels-Alder reaction of the diene 79 with benzyl nitrosoformate 7252 takes place at low temperature to afford the cycloaddition product in 54% yield as a single diastereoisomer. This adduct 80 was transformed into enantiomerically pure 1,4,5-trideoxy-1,4-imino-L-ribitol 81 (Figure 26).
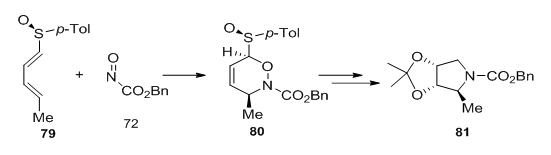
Figure 26 Adduct 80 was transformed into enantiomerically pure 1,4,5-trideoxy-1,4-imino-L-ribitol 81.
Addition reactions: Hydride reduction of the enamine with L- and K-Selectride displayed high diastereoselectivity (dr 95%) (Figure 27). The resulting compounds can be desulfinylated under reducing or Pummerer conditions to afford enantiomerically pure amines, aminoalcohols or aminoacids.
Takeda et al. have described a reductive cyclization to form functionalized cyclohexane derivatives that involves an intramolecular Michael addition.53 Hydride reduction of an enolate 85 afforded an ester enolate, which underwent intramolecular conjugate addition with vinylsulfoxide moiety to yield trans cyclization product 86 (Figure 28).

Figure 28 Hydride reduction of an enolate 85 afforded an ester enolate, which underwent intramolecular conjugate addition with vinylsulfoxide moiety to yield trans cyclization product 86.
Enantiomerically enriched sulfinyl auxiliaries are able to induce asymmetry in nucleophilic substitution reactions. Marino et al.54 demonstrated highly regio- and stereoselective SN2 reactions of enantiomerically enriched epoxy vinyl sulfoxide 87 with alkyl cyanocuprates to afford the hydroxy sulfoxides 88 and 89 (Figure 29). The displacements can either take place in syn or anti fashion, in which the sulfoxide is the predominant element of stereocontrol.

Figure 29 The displacements can either take place in syn or anti fashion, in which the sulfoxide is the predominant element of stereocontrol.
Steroid 11-oxoequilenin methyl ether 9055 was synthesized by stereospecific addition of the large (6-methoxy-2-naphthy1) magnesium bromide 91 to (S)-92 followed by trapping of the enolate intermediate with methyl iodide to yield 93 (Figure 30). Further elaboration gave optically pure (S,S)-90.
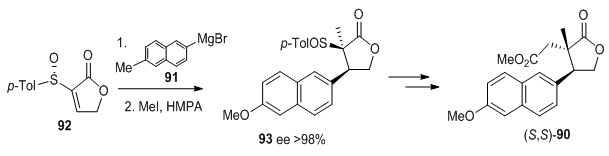
Figure 30 Steroid 11-oxoequilenin methyl ether 9042 was synthesized by stereospecific addition of the large (6-methoxy-2-naphthy1) magnesium bromide 91 to (S)-92 followed by trapping of the enolate intermediate with methyl iodide to yield 93.
While the intermolecular conjugate addition of nitrogen nucleophiles to α,β-unsaturated sulfoxides56 proceeds very slowly, the intramolecular Michael addition took place at lower temperature with higher reaction rates57,58 and modest asymmetric induction.
Compound 95, a key intermediate in the synthesis of α-tocoferol 9159 (vitamin E), was obtained by the addition of lithioalkenyl sulfoxide 92 to aromatic aldehyde 93 followed by intramolecular ring closure (Figure 31). A sole diastereomer was detected in the first reaction as well as in the intramolecular cyclization which took place via SN2 mechanism in a syn stereo specific fashion to give (2S)-95.
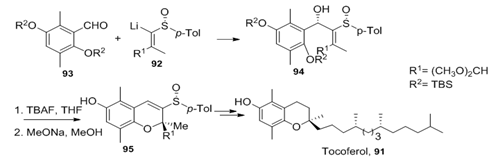
Figure 31 A sole diastereomer was detected in the first reaction as well as in the intramolecular cyclization which took place via SN2 mechanism in a syn stereospecific fashion to give (2S)-95.
Neighboring group participation of sulfoxides
The utility of sulfoxide as a neighboring group participant has been taken advantage of in the stereoselective synthesis of β-C-glycosides from 2-deoxy ribose.62 Acid catalyzed reaction of 2-deoxy-3-O-methyl sulfinyl ethyl ribofuranoyl acetate 99 with silyl enol ethers 100 and 101 proceeded stereo selectively resulting in the predominant formation of the corresponding β-C-glycosides 102 and 103 respectively (Figure 33).
Sulfinyl oxygen as a nucleophile in cyclic systems: The utility of the sulfinyl oxygen as a nucleophile was demonstrated by Montanari et al.63 in the synthesis of iodohydrin 104 from cyclic olefin 105. It was shown by 18O labeling studies that the sulfinyl oxygen participated as the nucleophile during the iodohydrin formation resulting in the Walden inversion at the sulfoxide center (Figure 34).
Sulfinyl oxygen as a nucleophile in acyclic systems: Sulfoxides have been used as pendant nucleophiles in the hydrolysis of epoxy sulfoxide 106 to dihydroxy sulfoxide 107. The sulfoxide delivered the oxygen intramolecularly (VII) to open the epoxide with high degree of regiocontrol.64 The reaction was postulated to proceed via the five membered cyclic sulfoxonium salts, which was hydrolysed to yield the dihydroxy sulfoxide (Figure 35).
Sulfinyl group participation was also demonstrated by Horner et al.65 in the stereoselective bromohydroxylation of 1,2-allenylsulfoxides with Br2 and H2O (Figure 36).
The potential of the sulfoxide group to act as an intermolecular nucleophile was demonstrated by David et al.66 A number of olefins were converted to bromohydrins stereospecifically by the use of N-bromosuccinimide (NBS) and moist dimethylsulfoxide (DMSO) (Figure 37).
With few exceptions, the sulfoxide group acts as an efficient chiral inducer in a number of organic transformations and has potential applications in natural product synthesis.67‒69 The key to the success is related to the steric and electronic differences between the substituents at the sulfur, as well as the conformational behavior of the sulfinyl group, which is able to react through a rigid conformation. In many cases use of Lewis acid could dramatically alter the stereoselectivity of the reaction. As, the sulfinyl functionality can co-ordinate both with its oxygen or sulfur atom extending the scope of metal used as catalyst. The field will probably be of growing interest in the near future.
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
There is no conflict of interest.

©2018 Sankaran, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.