MOJ
eISSN: 2574-819X


Review Article Volume 1 Issue 7
Department of Chemistry, Mody University of Science and Technology, India
Correspondence: Harshita Sachdeva, Department of Chemistry, College of Arts, Science and Humanities, Mody University of Science and Technology, Laxmangarh-332311 (Sikar), Rajasthan, India
Received: December 03, 2017 | Published: December 13, 2017
Citation: Sachdeva H, Khaturia S. A mini-review on organic synthesis in water. MOJ Biorg Org Chem. 2017;1(7):239-243. DOI: 10.15406/mojboc.2017.01.00041
The use of water as solvent in organic synthesis is one of the most powerful tool of green chemistry as it reduces emission of toxic chemicals in the environment thereby reducing pollution. Reactions can be carried out under mild conditions minimizing chemical waste with easy work up procedure enabling recycling of the catalyst. This review briefly highlights some important reactions carried out recently in water.
Keywords: green chemistry, water, organic synthesis, green solvent
Organic synthesis in water has attracted the attention of chemists for many years. Water is the nature’s solvent and possesses distinguished physical and chemical properties. It exhibits powerful hydrogen bonding and wide temperature range to remain in liquid state. In recent years, many organic transformations have been carried out in water.1,2 Many organic solvents like benzene, methanol, toluene are carcinogenic and can be toxic to human health and cause an environmental menace by polluting the atmosphere. The replacement of volatile organic solvents in organic reaction is an important aim of green chemistry.3,4 Earliest known examples of organic synthesis in water include Wohler’s urea synthesis and Baeyer and Drewsen’s indigo synthesis.5-7
In recent years, several N, O, and S containing heterocyclic compounds are synthesized employing green synthetic protocols. It is of considerable interest to learn more about varied green technology platforms, which have been utilized for the synthesis of important heterocyclic scaffold. Being an important and growing area of research, these green protocols have been regularly reviewed. The present attempt is to review briefly water mediated organic reactions, resulting in the synthesis of various heterocyclic frame works and to highlight the significance as well as utility of water as a green solvent.
Synthesis of substituted azo schiff bases8
Zarei M et al.8 reported the synthesis of pure azo Schiff bases (2) in high yields by mixing of the reagents either as aqueous slurry or by grinding at room temperature. Azoaldehyde (1) was prepared from p-anisidine and 2-hydroxy-3-methoxybenzaldehyde (o-vanillin) in aqueous medium at 0-5°C and then 1 was allowed to react with amines in a small amount of water at room temperature to produce azo Schiff bases (2) in excellent yields (Figure 1).
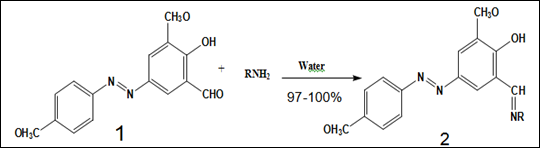
Figure 1 Synthesis of substituted azo Schiff bases.8
Synthesis of pyrano[2,3-c]pyrazoles9
Silica-water reaction medium was applied by Pravin et al.9 for the synthesis of various pyrano[2,3-c]pyrazole derivatives based on the adsorptive nature of silica. The reaction was carried out under mild and neutral conditions accepting several functional groups present in the molecules thus reducing the possibility of many unwanted side reactions. Present method offers marked improvements with regard to product yield, reaction time, and greenness of procedure, avoiding hazardous organic solvents/toxic catalysts and provides a better, clean and practical alternative to the existing protocols (Figure 2).
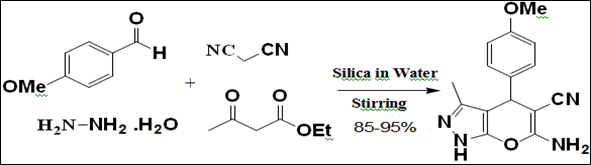
Figure 2 Synthesis of Pyrano[2,3-c]pyrazoles.9
Synthesis of aryldipyrromethanes10
Taoufik et al.10 have reported the oxidation of aryldipyrromethanes with p-chloranil (in dichloromethane), or DDQ (2,3-dichloro-5,6-dicyano-1,4-benzoquinone) at room temperature to obtain the corresponding dipyrrins in good yields (50-78%) (Figure 3).

Figure 3 Synthesis of aryldipyrromethanes.10
Synthesis of pyrimido[4,5-d]pyrimidine11
A practically convenient and eco-friendly aqua mediated synthesis of pyrimido[4,5-d]pyrimidines by the reaction of barbituric acid, aldehyde and urea or thiourea to yield pyrimido[4,5-d]pyrimidines has been developed by Kidwai et al.11 without using any catalyst and hazardous organic solvents and corrosive acids or bases. Water-insoluble solid products obtained in short time are found to be essentially pure and in very high yield (Figure 4).
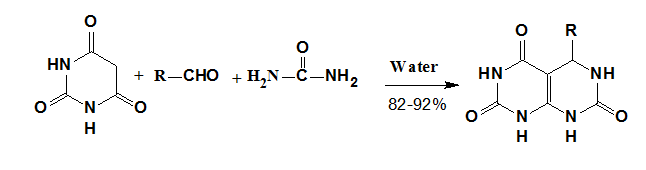
Figure 4 Synthesis of Pyrimido[4,5-d]pyrimidine.11
Synthesis of 2-aminothiazoles12
A highly efficient and facile method has been described by Taterao et al.12 for the synthesis of substituted 2-aminothiazoles in water without any added catalyst or co-organic solvent. The reaction was carried out at ambient temperature and the products were obtained in excellent isolated yields. The developed protocol is successfully applied for the preparation of an anti-inflammatory drug, fanetizole (Figure 5).
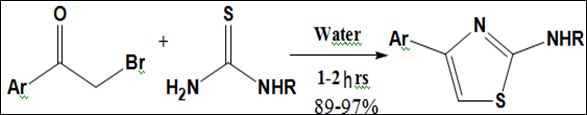
Figure 5 Synthesis of 2-aminothiazoles .12
Synthesis of alkyl or aryl-14H-dibenzo [a, j]xanthenes13
Alkyl- or aryl-14H-dibenzo [a,j]xanthene derivatives were synthesized efficiently by the reaction of beta-naphthol and aliphatic and aromatic aldehydes in the presence of KAl(SO4)2 x 12 H2O (alum) under aqueous condition at 100°C by Dabiri et al.13 Different types of aromatic and aliphatic aldehydes were used in the reaction and in all cases the products synthesized successfully. Several solvents were examined for this reaction; however, in terms of reaction yield and time, water was found to be the optimum solvent (Figure 6).
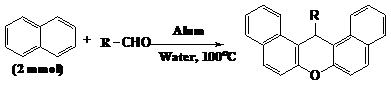
Figure 6 Synthesis of alkyl or aryl-14H-dibenzo [a, j]xanthenes .13
Syntheses of N-azacycloalkanes, pyrazole, pyrazolidine, and phthalazine derivatives14
Ju et al.14 have reported the synthesis of nitrogen-containing heterocycles from alkyl dihalides (ditosylates) and primary amines and hydrazines via a simple and efficient cyclocondensation in an alkaline aqueous medium that occurs under microwave irradiation. This improved greener synthetic methodology provides a simple and straightforward one-pot approach to the synthesis of a variety of heterocycles, such as substituted azetidines, pyrrolidines, piperidines, azepanes, N-substituted 2,3-dihydro-1H-isoindoles, 4,5-dihydropyrazoles, pyrazolidines, and 1,2-dihydrophthalazines (Figure 7).
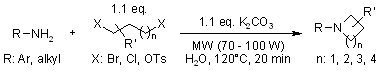
Figure 7 Syntheses of N-Azacycloalkanes, Pyrazole, Pyrazolidine, and Phthalazine derivatives.14
Synthesis of pyrazoles and diazepines15
Polshettiwar et al.15 have reported an expeditious room temperature synthesis of pyrazoles and diazepines by condensation of hydrazines/hydrazides and diamines with various 1, 3-diketones. This greener protocol was catalyzed by polystyrene supported sulfonic acid (PSSA) and preceded efficiently in water in the absence of any organic solvent within 1-2 min (Figure 8).
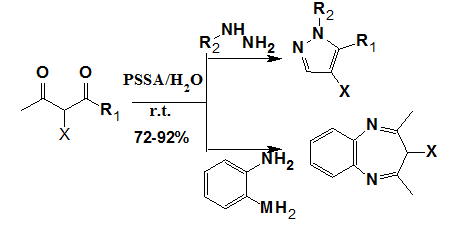
Figure 8 Synthesis of pyrazoles and diazepines.15
Synthesis of Substituted 3,4-dihydropyrimidin-2(1H)-ones16
An environmentally benign aqueous Biginelli protocol for the synthesis of substituted 3,4-dihydropyrimidin-2(1H)-ones using polystyrenesulfonic acid (PSSA) as a catalyst has been achieved by Polshettiwar et al.16 These microwave-assisted reactions proceed efficiently in water in the absence of organic solvent, with simple filtration as the product isolation step (Figure 9).
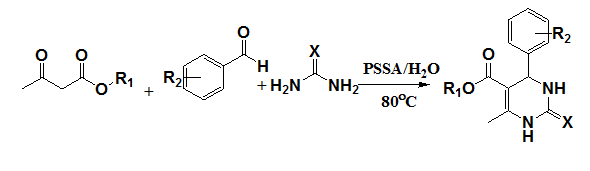
Figure 9 Synthesis of substituted 3,4-dihydropyrimidin-2(1H)-ones.16
Synthesis of 1, 3 dioxanes17
A novel tandem bis-aldol reaction of ketone with paraformaldehyde catalyzed by polystyrenesulfonic acid is reported by Polshettiwar et al.17 in aqueous medium to yield 1,3-dioxanes. This one-pot, operationally simple microwave-assisted synthetic protocol proceeds efficiently in water in the absence of any organic solvent, with excellent yield (Figure 10).
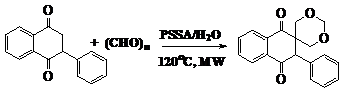
Figure 10 Synthesis of 1, 3 dioxanes.17
Synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates18
A green and efficient one-pot, four-component synthesis of methyl 6-amino-5-cyano-4-aryl-2, 4-dihydropyrano [2, 3-c] pyrazole-3-carboxylates in water is reported by Adeleh et al.18 The method is catalyst-free, atom-economical, and does not involve tedious work-up or purification affording the target compounds in good yields (Figure 11).
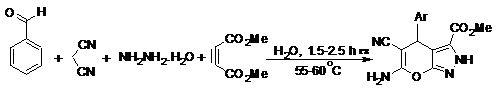
Figure 11 Synthesis of methyl 6-amino-5-cyano-4-aryl-2,4-dihydropyrano[2,3-c]pyrazole-3-carboxylates.18
Synthesis of coumarins19
A simple and convenient method for the efficient synthesis of coumarins in water catalyzed by K2CO3 is developed by Rehanaanjum.19 Optimal result was obtained using 20 mol% of K2CO3. The experiment was conducted with phenolic substrate and β-keto ester in the presence of catalytic amount of K2CO3 (20 mol %) in water. The reaction proceeded spontaneously at ambient temperature and was completed within 1 hr. The precipitated solid was filtered, dried and washed with 20 % ethyl acetate in petroleum ether to afford coumarins (Figure 12).
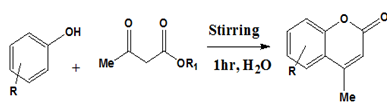
Figure 12 Synthesis of Coumarins.19
Synthesis of 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H chromene-3-carbonitrile derivatives20
Kumaravel et al.20 have developed a catalyst-free combinatorial library of novel 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4H-chromene-3-carbonitrile derivatives by the reaction of hydrazine hydrate, ethyl acetoacetate, 2-hydroxybenzaldehydes and malononitrile in water at ambient temperature (Figure 13).
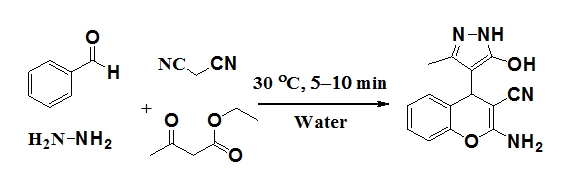
Figure 13 Synthesis of 2-amino-4-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-4Hchromene-3- carbonitrile derivatives.20
Synthesis of spiroindoline-pyranopyrazoles21
Ahadi et al.21 have reported the synthesis of spiro[indoline-3,4′-pyrano[2,3-c]pyrazole]-5′-carbonitriles with high yields and purity by the one-pot, four-component reaction of β-ketoesters, hydrazine hydrate, malononitrile, and isatins in aqueous media at room temperature (Figure 14).
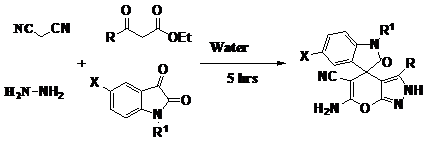
Figure 14 Synthesis of spiroindoline-pyranopyrazoles.21
Synthesis of 2-amino-1, 3, 4-thiadiazoles22
Aryanasab F et al.22 have reported a new and facile protocol for the synthesis of 2-amino-1,3,4-thiadiazoles in water by the reaction of acid hydrazides with dithiocarbamates in moderate to excellent yields (Figure 15).
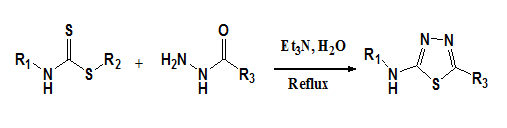
Figure 15 Synthesis of 2-amino-1, 3, 4-thiadiazoles.22
Synthesis of novel thiochromone-annulated thiopyranocoumarin derivatives23
Moghaddam et al.23 have reported an efficient catalyst-free synthesis of novel pentacyclic thiochromone-annulated thiopyranocoumarin derivatives via domino Knoevenagel-hetero-Diels-Alder reaction of 4-hydroxy dithiocoumarin and O-acrylated salicylaldehyde derivatives in H2O as solvent. The products are formed in good yields with high regio- and stereo-selectivity (Figure 16).
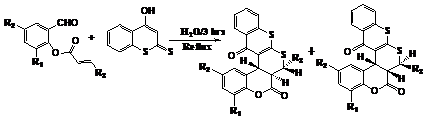
Figure 16 Synthesis of novel thiochromone-annulated thiopyranocoumarin derivatives.23
Synthesis of 3, 4-dihydroquinoxalin-2-amine derivatives24
A simple and efficient one-pot synthetic approach for the preparation of biologically interesting 3,4-dihydroquinoxalin-2-amine derivatives using EDTA-catalyzed three component reactions of o-phenylenediamines, carbonyl compounds, and isocyanides in an aqueous medium is described by Kolla et al.24 This method is of great value because of its environmentally benign character, high yields, and ease of handling (Figure 17).
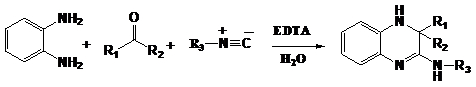
Figure 17 Synthesis of 3, 4-dihydroquinoxalin-2-amine derivatives.24
Synthesis of substituted fused thiazole derivatives25
Alison et al.25 have reported that hetero-cycloisomerization reactions of propargylic alcohol derivatives leading to indolizines have been demonstrated to proceed in the presence of water alone. This stands as a significant advance over the previous methods using Pt, Cu or Ag salts paired with ligands in organic solvents such as benzene, acetonitrile or methylene chloride (Figure 18).
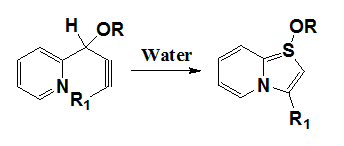
Figure 18 Synthesis of substituted fused thiazole derivatives.25
Gawande26 provided a brief overview of some selective catalyst-free reactions in aqueous media.26 Further, many organic molecules are partially soluble in water and reactions that appear as heterogeneous mixtures and suspensions may involve on-water and in-water reaction modes occurring simultaneously. Organic synthesis reactions on water at the organic-liquid water interface were studied by Butler et al.27 García-García et al.28 have reported the preparation of a mesoscopic metal-organic structure based on single-layer assembly of aluminium chains and organic alkylaryl spacers. The material markedly accelerates condensation reactions in water in the absence of acid or base catalyst, as well as organocatalytic Michael-type reactions that also show superior enantioselectivity when comparing with the host-free transformation.28 Alaoui et al.29 have reported the ultrasound assisted facile one pot synthesis of sulfonamide-isoxazoles using cerium (IV) ammonium nitrate (CAN) as an efficient oxidant in aqueous medium.29 Dilauro et al.30 have reported the nucleophilic additions of highly polar organometallic compounds to imines and nitriles using water as a reaction medium.30 They reported that the addition of highly polar organometallic compounds to non-activated imines and nitriles proceeds quickly, efficiently, and chemoselectively with a broad range of substrates at room temperature and under air with water as the only reaction medium.
In this review article, we have focused on some aqua mediated organic transformations which will surely help scientists and researchers across the globe to develop new inexpensive, clean and environmentally benign strategies to carry out organic synthesis.
The authors are thankful to the Dean, Prof. Atul Kumar, CASH, MUST, Laxmangarh (Sikar), Rajasthan, India for providing support and necessary research facilities in the department.
The author declares no conflict of interest.

©2017 Sachdeva, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.