MOJ
eISSN: 2574-819X


Technical Paper Volume 2 Issue 3
Department of Chemistry, Drexel University, USA
Correspondence: Hai-Feng Ji, Department of Chemistry, Drexel University, Philadelphia, PA 19104, USA, Tel 8564 1876 31
Received: May 09, 2018 | Published: June 11, 2018
Citation: Johnson NM, Ji H. Conversion of 2,3,8,9-dibenzo-4,7-Dimethyl-5,6-dihydro-1,10 phenanthroline to Dibenzo [b,j][1,10] phenanthrolines. MOJ Biorg Org Chem. 2018;2(3):154-157. DOI: 10.15406/mojboc.2018.02.00072
In this paper, we investigated and explained the low conversion efficiency of 2,3,8,9-dibenzo-4,7-Dimethyl-5,6-dihydro-1,10-phenanthroline (2) to 5,8-dimethyl-Dibenzo[b,j][1,10] phenanthrolines (1) and the reactivity of the 1. Chemistry from similar systems predicted a generally unreactive system, with any reactivity centered at the heteroaromatic rings. Several oxidized products from 2 were also reported. Keywords: 5,8-dimethyl-Dibenzo[b,j][1,10] phenanthrolines, carboxylic acids, aldehydes, carboxylic acids, and ketones, pyridinium chlorochromate, napthalene, anthracene, tetracene, pentacene
NMR, nuclear magnetic resonance; GAMESS, general atomic and molecular electronic structure system; PAHs, polycyclic aromatic hydrocarbons; DSSCs, dye-sensitized solar cells; DFT, density functional theory
One of the practical approaches to improve the efficiency of Dye-Sensitized Solar Cells (DSSCs) is to increase the number of absorbed photons. However, the absorption spectrum for most of the commonly used dyes is rather weak through the visible and infrared region and the solar spectrum flux is the greatest in this region. Therefore, a great deal of research has been focused on increasing the overall absorption, with a specific emphasis on an overall redshift. This can usually be accomplished by creating an extended p-system, which often serves both purposes simultaneously. However, while there have been many, many publications on extending the p-system through conjugation of aromatic systems,1 there have been relatively few that work by expanding the aromatic system.2 Those few attempts have shown very promising results in the shift in the absorption spectrum, however, the overall efficiency was quite low. In our work, we attempted to create a new ligand for Ru-based DSSCs, using an extended aromatic system with a backbone of 5,8-dimethyl-dibenzo[b,j ][1,10] phenanthroline.
5,8-dimethyl-Dibenzo[b,j][1,10] phenanthrolines1 (Figure 1) is a challenge compound to synthesis. It has the potential to combine the properties of polycyclic aromatic hydrocarbons (PAHs) with the flexibility of coordination complexes and the possibility to convert methyl groups to other functional groups for various applications. Dibenzo[b,j][1,10] phenanthrolines have been used in macrocycles to inhibit telomerases3 and destabilize DNA,4 can activate nucleases when used in copper (II) complexes,5 and have been shown to readily form coordination complexes with ruthenium.6 However, their applications are rather limited, potentially by the low synthetic yield and lack of knowledge about their fundamental chemistry.
The synthesis of 1 was reported by Kempter and Stoss.7 It involved a Friedländer condensation, followed by a palladium-catalyzed dehydrogenation of the product 2,3,8,9-dibenzo-4,7-Dimethyl-5,6-dihydro-1,10-phenanthroline (2). The yields for the palladium-catalyzed dehydrogenation (Figure 2) were quite low (10-20%). Conditions for this reaction relied on elevated temperatures (240°C), which have a high potential for unwanted side reactions on a compound of this type, potentially explaining the low yield.
In this work, we first tried the oxidative dehydrogenation approach to make 1, and then investigated the temperature effect in order to understand why the reaction yield is low. A potential method on similar systems to generate compound 1 from 2 is oxidative dehydrogenation from 2. This technique can be done with a number of different oxidizing agents.8‒10 Since the methyl group in 2 can be readily oxidized, mild oxidizers were used. When SeO2 was used, the reaction resulted 3 and 411 (Figure 3). This was quite successful in dehydrogenation the central bond, but only after oxidizing the methyl groups. 3 can be isolated after 4 hrs of exposure. Using more equivalents of SeO2 and reacting overnight forms 4 in high yields. Allowing the reaction to continue with an excess of SeO2 over two days begins to oxidize the aldehydes to carboxylic acids.
When MnO2 was used, the reaction formed a complex mixture of aldehydes, carboxylic acids, and ketones, with none of the target compound (1) found. Using mild chromium oxidizing agents such as Jones’ reagent and pyridinium chlorochromate leads to the simultaneous formation of the unusual side product of a 6,7-dione (5),12 indicating a very reactive olefinic bond (Figure 4). This was followed by rapid oxidation of the methyl groups to aldehydes and acids, as seen with MnO2. The three conditions are summarized in Table 1.
To understand why the oxidative dehydrogenation cannot be used for the synthesis of 1 from 2, we revisited the dehydrogenation reaction with the palladium catalyst under lower temperature. The results showed that no conversion was seen at lower temperatures in methanol, toluene, xylenes, 1,2-dichlorobenezene, acetic acid, and decalin. Decomposition was seen in nitrobenzene, and limited yield (10%) was seen in isocetane.
Both sets of experimental results indicate that 1 would be highly unusual based on what we know about PAH reactivity. No conversion was seen at lower temperatures indicates a high activation energy barrier for the reaction, which wouldn’t be expected from the planarity and aromatic nature of the target compound. Napthalene, anthracene, tetracene, and pentacene13 (Figure 5) are all readily formed from their various dihydro derivatives in boiling xylene, which indicates that molecular size and distribution of aromaticity are not directly relevant to the activation energy barrier.13 However, the increase in temperature from phenanthrene (110⁰C)14 to pentaphene (180°C)15 indicates that the branched nature of the system is directly relevant to this activation energy barrier.
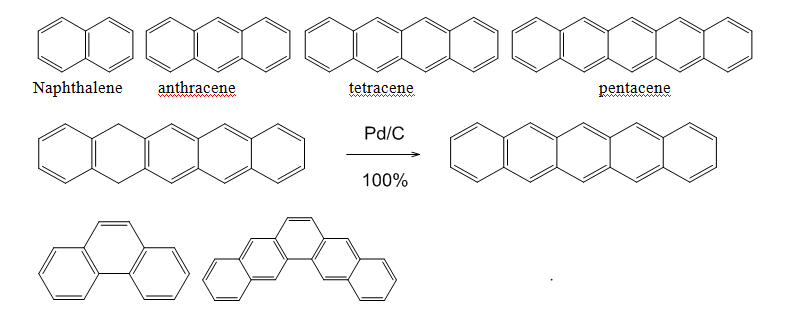
Figure 5 Molecular structures of several PAHs and an example of conversion to arene from it dihydro derivative.
Phenanthrene Pentaphene
As the catalytic surface shouldn’t distinguish between a linear and a curved compound, this would indicate that the branched nature of the system directly impacts the aromaticity of the central double bond. The reactivity of PAHs is generally governed by Clar’s rule,16 which states that aromaticity in the PAHs can be localized to those six-membered rings which contain a complete π sextet. Whichever resonance structure contains the most of these complete π sextets is known as the Clar structure, and will determine the properties of the molecule. Based on this, it is believed that compounds such as phenanthrene and phenanthroline have a sort of olefinic double bond property at the central position, while acridine and anthracene (Figure 6) would have aromaticity spread through the entire compound. According to Clar’s rule, very little aromatic stabilization is gained by this dehydrogenation of 2 to 1.
We also conducted a DFT (Density Functional Theory) calculations (Figure 7) of 2, which show that in the HOMO, there is a general lack of electron density in the system, except for at the nitrogen atoms, which would support a generally π deficient system. The lack of electron density in the HOMO at the central double bond also suggests low reactivity of the bond for oxidative dehydrogenation.
Energy minimization was initially done using an MMF94 force field. DFT calculations were done using the B3LYP/6-31G* basis set, with the General Atomic and Molecular Electronic Structure System (GAMESS) package in the ChemBio3D software. All reactions were performed under nitrogen unless specified otherwise. All chemicals were purchased from Fisher Scientific and were used as received. Deuterated solvents were purchased from Across Organics. Nuclear Magnetic Resonance (NMR) spectra were obtained on Varian INNOVA 300 MHz and 500 MHz NMR spectrometers.
6,7-dihydrodibenzo[b,j][1,10] phenanthroline-5,8-dicarbaldehyde (3)
Into a round-bottomed flask was added 312.5mg (1.01mmol)2, 275.3mg (2.50 mmol) of freshly sublimed SeO2, and 40mL of wet 1,4-dioxane. This was refluxed for 4hours. The resulting red suspension was filtered through Celite, and the filtrate was concentrated 50% under vacuum. The solution was then diluted with ether, and the precipitate was filtered and washed with acetone and ether to yield 274.5mg (81%) of product. 1H NMR (500 MHz, Chloroform-d) δ 11.15 (d, J=1.0 Hz, 2H), 8.59 (d, J=8.9 Hz, 2H), 8.53 (d, J=8.7 Hz, 2H), 7.83 (ddd, J=8.4, 6.9, 1.4Hz, 2H), 7.74 (ddd, J=8.2, 6.8, 1.3 Hz, 2H), 3.61 (d, J=1.1 Hz, 5H).
Dibenzo[b,j][1,10] phenanthroline-5,8-dicarbaldehyde (4)
Into a round-bottomed flask was added 310.7mg (1.01mmol) of 1, 267.1mg (2.41mmol) of freshly sublimed SeO2, and 40mL of wet 1,4-dioxane. This was refluxed for 16hours. The resulting red suspension was filtered through Celite, and the filtrate was concentrated 50% under vacuum. The solution was then diluted with ether, and the precipitate was filtered and washed with acetone and ether to yield 292.4mg (87%) of product. 1H NMR (498 MHz, Chloroform-d) δ 11.54 (s, 2H), 8.79 (t, J=7.9 Hz, 4H), 8.68 (s, 2H), 8.00 (td, J=8.6, 7.3, 1.6 Hz, 2H), 7.88 (td, J=7.7, 1.6 Hz, 2H).
3.3. 5,8-dimethyldibenzo[b,j][1,10]phenanthroline-6,7-dione (5)
450.1mg (1.53mmol) of K2Cr2O7 was dissolved in 5mL of H2SO4. This solution was then slowly added to a vigorously stirred solution of 156.2mg (0.504mmol) of 2 in 20mL of acetone. After 2hours, the green solution was diluted with water, filtered and washed with water and aqueous NaHCO3. The solid was then chromatographed on neutral alumina (80:20 ethyl acetate/hexanes) to yield 155.3mg of yellowish solid (30% yield). 1H NMR (499 MHz, DMSO-d6) δ 8.76 (d, J=4.3Hz, 2H), 8.11 (dd, J=8.4, 1.3Hz, 2H), 8.03–7.99 (m, 4H), 7.76 (ddd, J=8.3, 6.9, 1.4 Hz, 2H), 7.64 (ddd, J = 8.2, 6.8, 1.3 Hz, 2H), 7.41–7.36 (m, 1H), 2.69 (d, J = 0.9 Hz, 6H).
Our results indicate an unusual reactivity for 2 and 1. Low conversion efficiency of 2 to 1 was explained. Chemistry from similar systems would predict a generally unreactive system, with any reactivity centered at the heteroaromatic rings.
None
The authors declare no competing financial interest.

©2018 Johnson, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.