MOJ
eISSN: 2574-819X


Mini Review Volume 2 Issue 2
1Department of Pharmaceutical Chemistry, Aden University, Yemen
2Department of Pharmaceutical Chemistry, Anadolu University, Turkey
Correspondence: Weiam Hussein, Anadolu University, Faculty of Pharmacy, Department of Pharmaceutical Chemistry, Eski-ehir, Turkey, Tel +905074929053
Received: February 23, 2018 | Published: March 12, 2018
Citation: Hussein W, Zitouni TG. Synthesis of new thiazole and thiazolyl derivatives of medicinal significant-a short review. MOJ Biorg Org Chem. 2018;2(2):52-55 DOI: 10.15406/mojboc.2018.02.00056
In the field of therapeutic science, thiazoles are of extraordinary importance, because of their potent and significant biological activities. Likewise thiazole and their derivatives are found in various powerful naturally and biologically active compounds which possess a broad spectrum of biological activity therefore, synthesis of this compound is of remarkable concern. This review primarily focuses on the updated research papers reported in literature for the synthesis of thiazole and thiazolyl compounds.
Keywords: thiazoles, thiazolyl, synthetic procedures, biological activity
The synthesis of heterocyclic rings has been a fascinating field in therapeutic science. Various heterocyclic compounds containing nitrogen and sulfur have flexible frameworks for drugs development and design.1 Thiazole is one of the most intensively studied classes of aromatic five-membered heterocyclics. It was first defined by Hantzsch and Weber in 1887. Thiazoles are important class of heterocyclic compounds, found in many powerful biologically active drugs such as Sulfathiazol (antimicrobial drug), Ritonavir (antiretroviral drug), Abafungin (antifungal drug) and Tiazofurin (antineoplastic drug).2 Thus, thiazole or thiazolyl moiety if it is present in any compound will show numerous biological activities such as antimicrobial & antifungal,3 anti-inflammatory,4 anticancer,5 antihypertensive,6 anti-HIV,7 anticonvulsant8 and antidiabetic9 activities. Accordingly, this imperative ring framework keeps on pulling in consideration from the medicinal chemists and new synthetic way for the development of this heteroaromatic ring keeps on being developed. This short review mainly focuses on the research work reported in the recent literatures on various synthetic methods of thiazole and thiazolyl compounds.
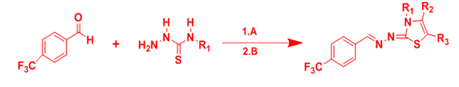
Santana et al.10 reported the synthesis of new substances belonging to thiosemicarbazones and thiazoles to develop powerful therapeutic agents for cancer treatment. The intermediate thiosemicarbazones were prepared in reaction between the 4'-trifluoromethyl-benzaldehyde with the corresponding thiosemicarbazide under reflux and catalytic amount of HCl. These intermediate compounds then react with different α-halogenated ketones, to obtain the series with yields of 22% to 94% (Figure 1).
Kaplancikli et al.11 reported a simple and three steps-reaction procedure for the synthesis of new benzimidazole-thiazole derivatives. 4-(1H-benzimidazol-1-yl)benzaldehyde was prepared by reacting 1H-benzimidazole and 4-fluorobenzaldehyde under microwave irradiation, then, the reaction between 4-(1H-benzimidazol-1-yl)benzaldehyde and hydrazine carbothioamide gave as a result 2-(4-(1H-benzimidazol-1-yl)benzylidene)hydrazine-1- carbothioamide derivatives which on reaction with appropriate 2-bromoacetophenones produced the final compounds (Figure 2).
Bikobo et al.12 synthesized a series of novel N-phenyl-4-(4-(thiazol-2-yl)-phenyl)thiazol-2-amine and 3-(2-(phenylamino)thiazol-4-yl)-benzamide ether derivatives First of all , 4-(a-bromoacetyl)-benzonitrile was refluxed with N-phenyl thiourea, to obtain 4-((2-phenylamino)thiazol-4-yl)-benzonitrile, which undergoes Willgerodt-Kindler reaction (FeS, TEA and HCl) producing H2S,to give of 4-((2-phenylamino)thiazol-4-yl)benzothioamide. This was then condensed with various halo-ketones in acetone or ethanol, to afford, N-phenyl-4-(4-(thiazol-2-yl)phenyl)thiazol-2-amine derivatives. In another case, N-phenylthiourea was heated with 2-hydroxy-5-(a-bromoacetyl) benzamide, and gave 2-hydroxy-5-(2-(phenylamino)thiazol-4-yl)benzamide , which was then treated with K2CO3, in acetone-DMF and stirred at room temperature, to give the corresponding potassium phenolate .The later one was treated with a-halo-ketones, in order to give the 5-(2-(phenylamino)thiazol-4-yl)benzamide ethers in good yields (72–96%) (Figure 3).
Farghaly et al.13 described the synthesis of new thiazole derivatives. The thiocroman-4-thiocarbohydrazone was used in this work as starting material for synthesis of new series of thiochromanes incorporating thiazole moiety by its reaction with a variety of hydrazonoyl chlorides using triethylamine as a catalyst (Figure 4).
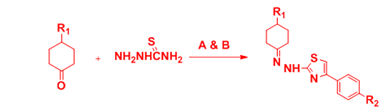
Turan-Zitouni et al.14 reported the synthesis of new 2-[2-[4-(ethyl/phenyl)cyclohexylidene]hydrazinyl]-4-(4-substitutedphenyl)thiazole by two step synthesis, thus the reaction of 4-(ethyl/phenyl) cyclohexanone with thiosemicarbazide resulted to the formation of 2-[4-(ethyl/phenyl)cyclohexylidene]hydrazine-1-carbothioamide derivatives . Then equimolar quantities of above mentioned compounds with appropriate phenacyl bromide in ethanol as solvent resulted to the formation of the final compound (Figure 5).
Tabbi et al.15 Synthesized new thiazolylpyrazoline derivatives by reacting substituted 3,5-diaryl-1-thiocarbamoyl-2-pyrazolines with phenacylbromides. The intermediate products were synthesized via the base catalyzed Claisen–Schmidt condensation. All the synthesized compounds displayed good antibacterial activity against K. pneumoniae and P. aeruginosa, with a MIC value of 200µg/mL (Figure 6).
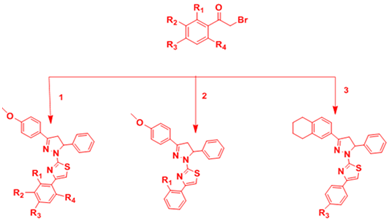
1.3-(4’-Methoxyphenyl)-5phenyl-2-pyrazolin-1-carbothioamide
2.3-(4’-methoxyphenyl)-5-phenyl-1-thiocarbomoyl-2-pyrazoline
3.5-phenyl-3-(5,6,7,8-tetrahydronaphthalen-2-yl)-1-thiocarbamoyl-2-pyrazoline
Figure 6 Synthesis of title compounds.
Gomha et al.16 reported the synthesis of new thiazolyl-thiazole derivatives by the reaction of 5-acetyl-2-amino-4-methylthiazole with thiocarbohydrazide and thiosemicarbazide in absolute ethanol and in the presence of a catalytic amount of concentrated HCl as indicated in Figure 7.
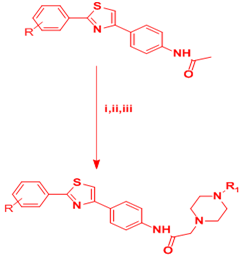
Yurttaş et al.17 described the synthesis of new 2-(4-arylpiperazine-1-yl)-N-[4-(2-(4-substituted phenyl)thiazol-4-yl)phenyl]acetamide derivatives by a five-step reaction, thus different 4-substituted thiobenzamides (H, OCH3, Cl, F) were used as starting materials. They reacted with N-[4-(2-bromoacetyl)phenyl]acetamide to obtain thiazole compounds. After deacetylation of these intermediates, 2-chloro-N-[4-(2-(4-substituted phenyl)-4-thiazolyl)phenyl]acetamides were obtained. Finally, 2-[4-(aryl)piperazin-1-yl]-N-[4-(2-(4-substituted phenyl)thiazol-4-yl)phenyl]acetamide derivatives were afforded by the reaction of these intermediates and corresponding piperazine derivatives. All these compounds were evaluated for their antimicrobial and anticholinesterase activities (Figure 8).
Özkayet al.18 synthesized some hydrazone derivatives of thiazole to evaluate their anticholinesterase activities. Pyrrole-2-carboxaldehydes reacted with thiosemicarbazide in ethanol then, the thiosemicarbazones obtained were condensed with α-bromoacetophenone derivatives (Hantzsch reaction) to give 1-substituted pyrrole-2-carboxaldehyde (4-(4-substituted phenyl)-1,3-thiazol-2-yl) hydrazones (Figure 9).
Penta et al.19 developed a novel one-pot synthesis of thiazoles and thiazolyl-pyrazole derivatives via multi component approach. The condensation of 3-(2-bromoacetyl)-4-hydroxy-6-methyl-2H-pyran-2-one, thiosemicarbazide and various carbonyl compounds gave corresponding thiazole and thiazolylpyrazole derivatives in good yields by using Hantzsch-Thiazole synthesis. In addition, this method does not include the use of organic solvents thus it is an environmentally friendly process (Figure 10).
Kaplancikli et al.20 described the synthesis 2-[[(benzoxazole/benzimidazole-2-yl)sulfanyl]acetylamino]thiazoles derivatives. Thus, in this study, 4-substituted-2-(chloroacetylamino) thiazole was prepared by reacting 2-amino-4-substituted-thiazole with chloroacetyl chloride. The reaction of 4-substituted-2-(chloroacetylamino)thiazole, benzimidazole/ benzoxazole-2-thiole , and anhydrous potassium carbonate in acetone gave the 4- substituted-2-[[(benzoxazole/benzimidazole-2-yl)sulfanyl]acetylamino]thiazole derivatives as final products (Figure 11).
The synthetic significance of thiazole derivatives have been increased much by their recent medicinal applications as anticancer, antifungal, anti-inflammatory, antihypertensive, anti-HIV, anticonvulsant and antidiabetic thus, thiazole chemistry has developed in steady state as we can see in many recent literatures.21,22 Based on this; many recent articles showed the use of palladium(II) acetate which catalyzes a really selective coupling of 4-substituted 2-aminothiazoles from vinyl azides and potassium thiocyanate, whereas iron(III) bromide supports the formation of 4-substituted 5-thiocyano-2-aminothiazoles.23 In addition, a copper-catalyzed reaction of oxime acetates with isothiocyanates gives various 4-substituted and 4,5-disubstituted 2-aminothiazoles under mild reaction conditions via copper-catalyzed N-O bond splitting, activation of vinyl sp2 C-H bonds, and C-S/C-N bond formations.24 Copper also, facilitated [3+1+1]-type condensation of oximes, anhydrides and potassiumthiocyanate (KSCN) to give thiazoles in very good yields under very mild reaction conditions.25,26 The uses of DABCO (1,4-diazabicyclo[2.2.2]octane) as an organocatalyst for synthesis of new thiazole derivatives in short reaction times and good to high yields was also reported.27 Due to reactivity of 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3- carbonitrile towards thioglycolic acid the latter was used with different chemical reagents to give many thiazole derivatives.
In the current years, numerous thiazole derivatives have been synthesized and subjected to fluctuate the biological activity of thiazole. In this article, we try to survey the simplest synthetic way for thiazole-based derivatives and feature their parts in new leads recognizable proof and medication disclosure. In this way the journey to investigate numerous more changes on thiazole moiety should be proceeded.
Authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
There is no conflict of interest.

©2018 Hussein, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.