MOJ
eISSN: 2574-819X


Research Article Volume 2 Issue 2
1Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, India
2DNASkew Analytics Private Limited, India
3Department of Pharmaceutical Chemistry, Kamla Nehru College of Pharmacy, India
Correspondence: Debarshi Kar Mahapatra, PhD, Assistant Professor, Department of Pharmaceutical Chemistry, Dadasaheb Balpande College of Pharmacy, Rashtrasant Tukadoji Maharaj Nagpur University, Nagpur 440037, Maharashtra, India, Tel +9183 5782 1881, +9191 7232 2500, +9171 2251 5517
Received: February 01, 2018 | Published: March 9, 2018
Citation: Mahapatra DK, Das D, Shivhare RS, et al. Murrayanine-hydantoin and -thiohydantoin analogs as promising anti-convulsant agents: synthesis, characterization and molecular docking studies. MOJ Biorg Org Chem. 2018;2(2):46-50. DOI: 10.15406/mojboc.2018.02.00055
Based on the fact that three scaffolds; viz. murrayanine, Schiff’s base, and hydantoin have been reported to exhibit potent anti-convulsant activity, therefore, all three of them were integrated to shape a hybrid molecule which is believed to demonstrate excellent anti-convulsant activity owing to the incorporation of scaffolds. The present research involved rational designing of anti-convulsant agents having murrayanine scaffold linked with hydantoin moiety via Schiff’s base linkage with an objective that the analogs will demonstrate enhanced anti-convulsant activity than their parents and also show comparable activity with the standard drug. The anti-convulsant activity was screened at dose levels of 30, 100, and 300 mg/kg utilizing maximal electroshock-induced seizure (MES) threshold test where molecule (5) displayed the most potent activity at doses 30 mg/kg (0.5 hr) and 100 mg/kg (4 hr), respectively, with comparison to the standard drug, phenytoin. In contrast, the compound (8) compound exhibited activity at 100 mg/kg (0.5 hr) and 300 mg/kg (4 hr), respectively in male Albino Swiss mice. The research revealed the prospective of murrayanine-Schiff’s base-hydantoin derivatives as active anti-convulsant activity. The characterization data were found to be in full agreement with the structural aspects. The molecular docking study revealed the possible interaction of the ligands with the NaVAb voltage-gated sodium channel, thereby proving the possible mechanism of molecule action. This study will certainly promote researchers in the rational synthesis of hybrid molecules with pronounced anti-epileptic activity.
Keywords: murrayanine, schiff’s base, imdiazolidine-2,4-dione, hydantoin, anti-convulsant, epilepsy
Epilepsy is the oldest neurological disease of the brain characterized by hypersynchronous neuronal firing and hyperexcitability that finds recognition in ancient medical texts, dated 3000 years back.1 Presently, nearly 50 million people across the world are affected at all ages in low- and middle-income countries 35% of the patient population experience uncontrolled and associated seizures.2 Almost 60% of the population in under-developed nations is devoid of full-course medication facility, which in the due course leads to higher social discrimination. It is expected that by the end of the year 2020, 200 thousand more cases will arrive.3 The present treatment approach involves pharmacotherapeutics, clinical, and surgical approaches, which in the long run often results in several adverse effects.4 In comparison to drug discovery and utility, nearly 25 anti-epileptic drugs have arrived, which nearly respond to about three-fourth of the epileptic patients.5 However, neurotoxic nature, poor patient compliance, unpredictable pharmacokinetic attributes, and side-effects like drowsiness, anemia, ataxia, nausea, gastrointestinal disturbance, and hyperplasia have given rise to the need of discovering safe molecules with enhanced therapeutic regimen and equi-efficacious nature.6
In search for the novel molecules having enhanced therapeutic activity accompanied by lower neurotoxicity, a number of naturally occurring products of heterocyclic classes like flavonoids, chalcones, glycosides, etc. were screened. Although, they have gained immense reputation as anti-convulsant agents, but their use as first line ethno product is restricted due to their non-standardization.7 Numerous plant extracts have also demonstrated impressive anti-seizure activity in the maximal electroshock seizure (MES) and pentylenetetrazole (PTZ) tests.8 Murraya koenigii L. or Curry tree (Ruta¬ceae) is an important traditional herb of Indian origin that has been in practice for centuries.9 It is well known for its ethno pharmacological importance in the management of epileptic episodes.10 Aqueous11 and ethanolic12 extract of curry leaves have demonstrated potent anti-convulsant activity owing to the presence of carbazole molecules.
Schiff’s base containing molecules is referred to those compounds having azomethine group (C=N), formed by the condensation of primary amines and active carbonyl groups.13 Schiff’s bases are well known for their anti-convulsant activity. Heterocyclic molecules like isatin,14 benzothiazol,15 quinazoline,16 etc. having a Schiff’s base linkage or a conjugation have demonstrated remarkable anti-convulsant activity. Hydantoin (or imdiazolidine-2,4-dione) is the most promising scaffold for the management of epilepsy. Phenytoin, the prototype remained the most successful drug across the world owing to its potential in limiting the recurring firing of action potentials evoked by a sustained depolarization and also slowing of the rate of recovery of voltage-activated Na+ channels from inactivation.17
Based on the fact that all the three scaffolds; viz. murrayanine, Schiff’s base and hydantoin have been reported to exhibit potent anti-convulsant activity, therefore, all three of them were integrated to shape a hybrid molecule which is believed to demonstrate excellent anti-convulsant activity owing to the incorporation of scaffolds (Figure 1). Thus, the present research involved rational designing of anti-convulsant agents having murrayanine scaffold linked with hydantoin moiety via Schiff’s base linkage with an objective that the analogs will demonstrate enhanced anti-convulsant activity than their parents and also show comparable activity with the standard drug.
Chemical and instrumentation
All chemical derivatives, solvents, and analytical grade reagents employed in synthesizing the products were procured from Merck, HiMedia, and Sigma-Aldrich. The melting points of the synthesized derivatives were measured on Perfit melting point apparatus. Thin layer chromatography was carried out using silica gel G-coated TLC plates (Merck). The FT-IR spectra were recorded on KBr discs on the IRAffinity-1 instrument. The 1H-NMR (400 MHz) spectra were recorded using Bruker spectrospin NMR DPX-300. The TMS (Sigma-Aldrich) was used as an internal standard. The 13C-NMR (300 MHz) was carried out using JEOL AL300 FTNMR instrument. The mass spectra were obtained on JEOL-JMS-DX 303 instrument. The elemental analyses were performed on Perkin-Elmer 240C analyzer. Electroconvulsiometer (HICON®, Grover Ent., India) was used for inducing seizures.
Animals
Wistar rats aged 5-7 weeks, average weight 180-260 g were used for the anti-convulsant study after prior approval from the Department Ethical Committee and CPCSEA (1389/a/10/CPCSEA). Animals were housed in clean polypropylene cages having 2 rats per cage under temperature controlled rooms (25–26ºC, humidity 50–55%, 12 hr light and dark) with proper hygienic conditions. Free access to water and standard rodent pellets was allowed.
Extraction of murrayanine
The murrayanine was extracted as per our previously mentioned protocol18 where using soxhlet apparatus, the powdered stem bark of M. koenigii was extracted with n-hexane. The obtained extract was initially filtered through a cotton plug and successively with a Whatman filter paper. The concentrated plant extract was additional subjected to isolation by silica gel-based column chromatography technique using eluant mixtures in order; hexane, hexane/ethyl acetate, ethyl acetate, ethyl acetate/methanol and methanol to produce 75 fractions. All fractions were analyzed by TLC technique, where fractions B21-B37 of hexane extract were collectively taken to isolate the desired compound; murrayanine 1.
Synthesis of target compounds
The new analogs were fabricated from murrayanine 1, a carbazole moiety extracted skillfully from M. koenigii L. which already has remarkable anti-convulsant activity. With an objective and hope that the newly developed hybrid products will exhibit enhanced anti-convulsant activity than the parent and will have comparable activity like the standard anti-convulsant drug. In order to synthesize the desired compounds 5 and 8, the aldehydic group (-CHO) of murrayanine 1 was exploited profoundly to introduce a Schiff’s base function (C=N), which is also reported to exhibit notable anti-convulsant activity. The Schiff’s base was introduced by effortless reaction of murrayanine 1 with semicarbazide 2 to form an intermediate product 3. The thiosemicarbazide 6 was also made to react with murrayanine 1 to produce sulfur containing intermediate 7. The mechanism involved in this reaction is selective attack of the nucleophilic amine of 2 and 6 over electrophilic carbon atom of aldehydes group of 1 which ultimately results in the substitution of carbonyl by the azomethine. The last step of synthesis involved the cyclization of intermediate products 3 and 7 by ethyl chloroacetate 4 and fused sodium acetate in alcoholic media to produce hydantoin 5 and thiohydantoin 8 analogs of murrayanine. The Figure 2 describes the pathway involved in the synthesis.
1-methoxy-9H-carbazole-3-carbaldehyde (1)
m.p.: 165-167°C, Rf: 0.47, hexane: ethyl acetate: methanol (7:2:1). FTIR (KBr) υ (cm-1): 3250 (-NH), 3081 (C-H, aromatic), 1722 (C=O), 1295 (C-O). 1H NMR (δ, ppm, DMSO-d6): 10.4 (9, 1H), 9.81 (4, 1H), 7.2-8.8 (Aromatic, 6H), 3.86 (1, 3H); 13C NMR (δ, ppm, 300 MHz, CDCl3): 179.6 (3, Aldehyde), 105.7-129.2 (Aromatic), 57.4 (1, CH3). MS: [M+ 225; 181 (30%)]. Anal. Calcd. for C14H11NO2: C, 74.65; H, 4.92; N, 6.22. Found: C, 74.56; H, 4.88; N, 6.03.
Synthetic protocol for (E)-2-((1-methoxy-9H-carbazol-3-yl)methylene)semicarbazide (3)
Equal quantity (0.01 M) of murrayanine (1) and semicarbazide (2) was added gradually to the solution of ethanol and under continuous stirring, the content was refluxed for 12 hr. A few drops (5 to 7) of glacial acetic acid were added to the reaction mixture. The content was further cooled, the precipitate was collected, washed with ice cold water, dried properly and recrystallized with aqueous ethanol.14 63% yield; FTIR (KBr) υ (cm-1): 3423 (NH2), 3244 (NH), 3064 (C-H, aromatic), 1661 (C=N, azomethine), 1555 (-NH, bending), 1619 (C=C, aromatic), 1286 (C-O). 1H NMR (δ, ppm, CDCl3): 10.25 (9, 1H), 8.55 (azomethine, 1H), 7.3-8.5 (Aromatic, 6H), 6.14 (12, 1H), 3.91 (1, 3H); 13C NMR (δ, ppm, 300 MHz, CDCl3): 173.8 (13, C=O), 151.1 (10, Azomethine), 104.7-128.2 (Aromatic), 52.3 (1, CH3). MS: M+ 282. Anal. Calcd. for C15H14N4O2: C, 63.28; H, 5.00; N, 19.85. Found: C, 63.13; H, 4.93; N, 19.79.
Synthetic protocol for (Z)-3-((1-methoxy-9H-carbazol-3-yl)methyleneamino)imidazolidine-2,4-dione (5)
A mixture of (E)-2-((1-methoxy-9H-carbazol-3-yl)methylene)semicarbazide (3) (0.1 M), ethyl chloroacetate (0.1 M) (4) and fused sodium acetate (0.1 M) in ethanol, the mixture was heated under reflux for 6 h. The reaction mixture was cooled and poured into crushed ice. The resulting solid product was filtered, dried and recrystallized from absolute ethanol.
46% yield; FTIR (KBr) υ (cm-1): 3301 (-NH, stretch), 3105 (C-H, aromatic), 1688 (C=N, azomethine), 1640 (C=C, aromatic), 1551 (-NH, bending), 1293 (C-O). 1H NMR (δ, ppm, CDCl3): 10.17 (9, 1H), 9.19 (azomethine, 1H), 7.0-8.8 (Aromatic, 6H), 6.2 (Hydantoin, 1H), 4.14 (heterocyclic, 2H), 3.88 (1, 3H); 13C NMR (δ, ppm, 300 MHz, CDCl3): 173.1 (13, C=O), 168.5 (16, C=O), 153.2 (10, Azomethine), 106.7-131.3 (Aromatic), 59.9 (1, CH3). MS: M+ 322. Anal. Calcd. for C17H14N4O3: C, 63.35; H, 4.38; N, 17.38. Found: C, 62.99; H, 4.31; N, 17.08.
Synthetic protocol for (E)-2-((1-methoxy-9H-carbazol-3-yl)methylene)thiosemicarbazide (7)
Equal quantity (0.01 M) of murrayanine (1) and thiosemicarbazide (6) was added gradually to the solution of ethanol and under continuous stirring, the content was refluxed for 12 hr. A few drops (5 to 7) of glacial acetic acid were added to the reaction mixture. The content was further cooled, precipitate was collected, washed with ice cold water, dried properly and recrystallized with aqueous ethanol.
67% yield; FTIR (KBr) υ (cm-1): 3411 (-NH2), 3250 (-NH, stretch), 3076 (C-H, aromatic), 1664 (C=N, azomethine), 1613 (C=C, aromatic), 1567 (-NH, bending), 1285 (C-O), 1162 (C=S). 1H NMR (δ, ppm, CDCl3): 10.23 (9, 1H), 8.46 (azomethine, 1H), 7.1-8.6 (Aromatic, 6H), 3.97 (1, 3H), 2.2 (12, 1H); 13C NMR (δ, ppm, 300 MHz, CDCl3): 188.6 (13, C=S), 149.5 (10, Azomethine), 109.1-126.7 (Aromatic), 56.6 (1, CH3). MS: M+ 298. Anal. Calcd. for C15H14N4OS: C, 60.38; H, 4.73; N, 18.78. Found: C, 60.24; H, 4.70; N, 18.55.
Synthetic protocol for (Z)-3-((1-methoxy-9H-carbazol-3-yl)methyleneamino)-2-thioxoimidazolidin-4-one (8)
A mixture of (E)-2-((1-methoxy-9H-carbazol-3-yl)methylene)thiosemicarbazide (7) (0.1 M), ethyl chloroacetate (0.1 M) (4) and fused sodium acetate (0.1 M) in ethanol, the mixture was heated under reflux for 6 h. The reaction mixture was cooled and poured into crushed ice. The resulting solid product was filtered, dried and recrystallized from absolute ethanol.
39% yield; FTIR (KBr) υ (cm-1): 3285 (-NH, stretch), 3037 (C-H, aromatic), 1687 (C=N, azomethine), 1629 (C=C, aromatic), 1592 (-NH, bending), 1273 (C-O), 1184 (C=S). 1H NMR (δ, ppm, CDCl3): 10.33 (9, 1H), 9.27 (Azomethine, 1H), 7.2-8.7 (Aromatic, 6H), 6.1 (Hydantoin, 1H), 4.26 (heterocyclic, 2H), 3.80 (1, 3H); 13C NMR (δ, ppm, 300 MHz, CDCl3): 184.3 (13, C=S), 171.4 (16, C=O), 159.8 (10, Azomethine), 114.6-139.9 (Aromatic), 50.2 (1, CH3). MS: M+ 338. Anal. Calcd. for C17H14N4O2S: C, 60.34; H, 4.17; N, 16.56. Found: C, 60.09; H, 3.96; N, 16.21.
In silico molecular docking
The in silico docking experiment was executed as per the protocol performed in our previous research.19 All the compounds were first created by using Chem Draw Ultra v. 8.0 and energy was minimized by Merck Molecular Force Field application. The biological target NaVAb voltage-gated sodium channel (PDB ID: 3RVY) was acquired from RSCB and the ligands were docked by AutoDock 4.2, following the standard method for docking. The docking interactions were evaluated and expressed in terms of the binding score.20 The results of docking experiment were compared with a well-known sodium channel inhibitor phenytoin. Genetic Algorithm (GA) functions were implemented by MDS into the 3D model of the channel. An RMSD score lesser to or close to 2A° will be judged as a successful docking. 21
Anti-convulsant activity
Maximal electroshock method (MES) protocol, given by the National Institute of Neurological Disorders and Stroke, NIH (USA) was utilized for determining anti-convulsant activity of the two hybrid molecules. The standard drug, phenytoin sodium (30mg/kg i.p.) was believed to provide 100% protection against the convulsions. The convulsions were provoked by electroconvulsiometer by conveying 60 cycles of alternating current of intensity 50 mA for 0.25 seconds, through the corneal electrodes and administering 30, 100, and 300 mg/kg doses of hybrids. The rats were monitored for 30 min and the anti-convulsant activity was measured by the abolition of the hind limb tonic extensor spasm.22
Statistical analysis
The SEM (standard error mean) of the three observations were considerably diverse from the standard drug (ttab < tcal,) (student’s t-test; P < 0.05).
Chemistry
The elemental analyses represented the % estimation of the elements which were found to be in a very close conformity (in %) with that of the theoretical value(s). The characterization reports of the synthesized analogs by sophisticated analytical tools describe some striking features. The amide stretching was observed in the range of 3250-3423 cm-1 while the amide bending was detected in the range of 1551-1592 cm-1. Two important aspects of the key characteristics of aromatic ring were seen at the range 3037-3105 cm-1 which represented C-H stretching and in the range 1613-1640 cm-1 that symbolized C=C stretching. Schiff’s base azomethine(s) was recognized in the range of 1661-1688 cm-1 for the derivatives. In the case of 1H NMR, the amide protons were chiefly identified in the structure. The protons of the carbazole scaffold were identified in the range 10.1-10.3 ppm, the protons of (thio) semicarbazides as well as 5-membered hydantoin were identified at 6.0-6.2 ppm.
However, the intensity of the peaks was high in case of semicarbazide derivatives compared to hydantoin. The aromatic protons were located in the range of 7.0-8.8 ppm. Furthermore, the 13C-NMR spectra of the synthesized compounds revealed the key characteristic features of compounds. The carbon of the methyl group of carbazole was predominantly seen at 50.2-59.9 ppm. The aromatic carbons were primarily noticed at 106.7-139.9 ppm. The C=O at position-13, C=O at position 16, and C=S at position-13 of the 5-membered ring were principally differentiated by peaks at 173.1-173.8 ppm, 171.4 ppm, and 184.3-188.6 ppm, respectively. The conversions of murrayanine (1) to corresponding Schiff’s bases (3 and 7) were additional confirmed chiefly by 151.1 ppm and 149.5 ppm. The mass spectra presented the appearance of base peaks which were corresponding to the molecular weight of the analogs as well as several small fragment peaks in the m/z of >100.
In silico docking
The molecular docking of both the analogs (5 and 8) into the active site of NaVAb voltage-gated sodium channel revealed the possible affinity of the compounds towards the biological target where both the analogs interacted stalwartly with the amino acid residues. The highest docking score of -9.57 was observed for analog 5 which suggested the highest affinity for the channel, while the thio-containing analog 8 expressed docking score of -8.01. The docking score of the synthesized analogs is described in Table 1.
Compound |
Dock Score |
5 |
-9.57 |
8 |
-8.01 |
Table 1 Docking scores of the synthesized derivatives
Analog 5 exhibited a number of interactions, where the carbazole scaffold demonstrated pi-anion interaction with amino acids GLU177 and GLU180; and a pi-alkyl interaction were displayed with LYS177 residue. Several conventional hydrogen bonding was observed in the docking pose of the novel hybrid molecule. The azomethine nitrogen atom of the linker expressed an interaction with amino acid residue. The carbazole ring exhibited interaction with ASP181 amino acid of the channel; the oxygen atom of the hydantoin moiety showed interactions with TRP179 and GLU180 residues; and the hydrogen atom of the five membered ring illustrated interaction withTHR175 residue (Figure 3).
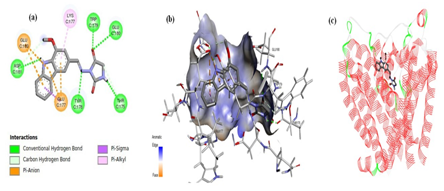
The docking pose of analog 8 displayed numerous interactions. The carbazole moiety showed three prominent interactions; Pi-alkyl interaction (with SER176 residue); pi-anion interaction (with ASP177 residue); and pi-sigma interaction (with ALA177 residue). Additionally, the methyl portion of OCH3 group of carbazole moiety expressed pi-sigma interaction. The oxygen atoms of OCH3 group and ring of carbazole portion and hydantoin moiety illustrated conventional hydrogen bonding with amino acid residues; GLU180 and LYS177, respectively. In contrast to analog 5, the azomethine nitrogen atom of the linker demonstrated no such interaction with any amino acid residue. The oxygen atom of the hydantoin moiety showed two prominent interactions; a carbon-hydrogen interaction with TRP179 and GLU180 residues; and the hydrogen atom of the five membered ring illustrated interaction withTHR175 residue (Figure 4).
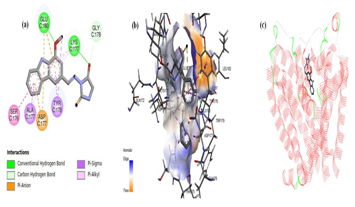
Anti-convulsant activity
The anti-convulsant activity was screened at dose levels of 30, 100, and 300 mg/kg utilizing maximal electroshock-induced seizure (MES) threshold test where molecule (5) displayed the most potent activity at doses 30 mg/kg (0.5 hr) and 100 mg/kg (4 hr), respectively, with comparison to the standard drug, phenytoin. In contrast, the compound (8) compound exhibited activity at 100 mg/kg (0.5 hr) and 300 mg/kg (4 hr), respectively in male Albino Swiss mice. Lipophilicity may be a key factor in exhibiting biological activity. The lower activity of the thio-based hybrid may be explained based on the high lipophilicity of the compound which might result in the distribution of the molecule from the CNS area. As per the observation in exhibiting CNS activity, a log P of >2.5 is needed,23 but higher values often lead to detrimental effects by migrating in the lipid rich sections of the body. The results were also found to be in close agreement with the computational observations. For that reason, the fabricated hybrids represented potent and comparable anti-convulsant activity than the standard. The anti-convulsant activity of the prepared compounds is summarized in Table 2.
Compounds |
MES Screening |
|
|
0.5 hr |
4 hr |
5 |
30mg/kg |
100mg/kg |
8 |
100mg/kg |
300mg/kg |
Phenytoin |
30mg/kg |
30mg/kg |
Table 2 Anti-convulsant and neurotoxicity evaluation of compounds
The research revealed the prospective of murrayanine-Schiff’s base-hydantoin derivatives as active anti-convulsant activity. The characterization data were found to be in full agreement with the structural aspects. The molecular docking study revealed the possible interaction of the ligands with the NaVAb voltage-gated sodium channel, thereby proving the possible mechanism of molecule action. This study will certainly promote researchers in the rational synthesis of hybrid molecules with pronounced anti-epileptic activity. The well-defined structure, biological data, computational outcomes, and a probable mechanism of action of the hydantoin linked hybrid of natural product may open new possibilities in designing better anti-convulsant agents in the near future.
Authors are highly thankful to Savitribai Phule Pune University, Pune, Maharashtra, India for providing research grants (Grant No. 13PHM000126).
Authors have no conflict of interest with the content of this article.

©2018 Mahapatra, et al. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.