MOJ
eISSN: 2574-819X


Mini Review Volume 2 Issue 2
Department of Pharmacy, Institute of Management and Technology, India
Correspondence: Mohammad Asif, Department of Pharmacy, GRD(PG) Institute of Management and Technology, Dehradun, 248009, Uttarakhand, India, Tel +9198 9708 8910
Received: March 01, 2017 | Published: March 7, 2018
Citation: Asif M, Antimicrobial potential of various substituted azetidine derivatives. MOJ Biorg Org Chem. 2018;2(2):36-40. DOI: 10.15406/mojboc.2018.02.00053
Azetidine derivatives are reported to show a variety of antimicrobial, antitubercular, anticonvulsant, anti-inflammatory and cardiovascular activities. This review is focused on antimicrobial activities of azetidine derivatives. The use of antimicrobial agents is limited due to rapidly developing drug resistance and side effects. Therefore, the development of new antimicrobial drugs is an essential aim. Much of research efforts are directed towards the design of new effective antimicribials.
Keywords: azetidinone, antimicrobial, antitubercular, antioxidant, anti-inflammatory
Many serious and life threatening diseases such as tuberculosis are caused by bacteria or fungi infections. Organ transplantation or surgery caused microbial infections are common problem.1,2 Natural, synthetic and semi synthetic antimicrobial drugs have been used against the life threatening infectious diseases.3 Deaths from bacterial and fungal infection have dropped currently, but still those are the major cause of death in the world.4 Over the few past decades the bacterial resistance to antibiotics, anti-fungal and anti-TB drugs has become one of the most challenging problems in the infections treatments. TB is a chronic grannulomatous disease5 and world’s oldest known infectious disease that causes about three million deaths each year. The causative organism of disease is Mycobacterium tuberculosis (Mtb).6 In the last decade, researchers made a continuous effort to fight these diseases and several new chemotherapeutic agents have been successfully introduced. Several azetidine containing drugs exhibited promising results.1,2 The urgency to develop new and effective drugs is due to the resistance development by strains against the current medications and growing problem of co-infection in immune-compromised patients.7,8 Therefore, more research affects that to develop new and better drugs against infections.9 The 2-Azetidinones are frequently encountered heterocycles in compounds of biological interest. They have been shown to possess a broad spectrum of biological activity.10 2-Azetidinone skeleton is well established as the pharmacophore of β-lactam antibiotics, the most widely employed class of antibiotics.11 The structural diversity of biologically active β-lactam antibiotics led to the development of methods for the construction of appropriately substituted azetidine with control of functional group and stereochemistry. The penicillin and cephalosporin antibiotics possess cis-β-lactam units, whereas the thienamycins and trinems have trans-β-lactam moieties. The synthesis of β-lactam became a desirable goal based on the discovery of penicillin and cephalosporin. Although most penicillin and cephalosporin related compounds are obtained by biosynthesis, chemical modification of intermediates for bioassay of the antibacterial activity of the resulting compounds has become of utmost importance because of the growing resistance of bacteria against penicillin-and cephalosporin-like compounds and the need for compounds with more specific antibacterial activity.10,11
Azetidine derivatives are reported to show a variety of antimicrobial, antitubercular, anticonvulsant, anti-inflammatory, antimalarial, anticancer, antiviral, antioxidant and cardiovascular activities.12‒24 These studies showed that even a minor change in the substitution pattern has a major effect on the activities of azetidine derivatives. The azetidinone derivatives have also been recognized as tumor necrosis factor-alpha (TNF-alpha) converting enzyme (TACE) inhibitors and agents with new biological activities, such as anticancer, anticoccidial, cardiovascular, antiviral, mutagenic, anticonvulsant and anti-inflammatory.25‒32 Some 2-oxo-azetidine derivatives of isoniazid have been tested for anti-bacterial, antifungal and anti-TB activity. The 4-oxo-Azetidines are four membered cyclic amides derived from Schiff bases which contain β-lactam unit as its essential structural feature of its molecule.33 The utility of 4-oxo-azetidines as synthons for various biologically active compounds, as well as their recognition as cholesterol absorption inhibitors and enzyme inhibitors has been studied.34
Various azetidines 1(a-m) (Figure 1) were tested for their antibacterial, antifungal and antitubercular activity which displayed acceptable results.
|
Compound |
Ar |
Compound |
Ar |
1a |
phenyl |
1h |
4-nitrophenyl |
|
1b |
4-chlorophenyl |
1i |
3-nitrophenyl |
|
1c |
3-chlorophenyl |
1j |
2-nitrophenyl |
|
1d |
2-Chlorophenyl |
1k |
4-methoyphenyl |
|
1e |
4-bromophenyl |
1l |
4-methylphenyl |
|
1f |
3-bromophenyl |
1m |
4-hydroxyphenyl |
|
1g |
2-bromophenyl |
|
||
Figure 1 Various azetidines 1(a-m) were tested for their antibacterial, antifungal and antitubercular activity.
The antibacterial, antifungal and antitubercular activities of compounds 1(a-m) have been assayed in vitro against selected Gram positive bacteria, Bacillus subtilis, Staphylococcus aureus and Gram negative bacteria, Escherichia coli, Klebsiella pneumoniae fungi, Aspergillus niger, Aspergillus flavus, Candida albicans Fusarium oxisporium and Mtb H37Rv strain, MIC values of compounds 1(a-m) were determined antibacterial, antifungal activities and antitubercular activity. Streptomycin and Griseofulvin were used as standard for antibacterial and antifungal activity and showed MIC range for all bacterial strain 1.25-6.25μg/mL, for all fungal strain activity was found to be 6.25-12.5μg/mL and for antitubercular activity, isoniazid and rifampicin taken as standards. Nitro group containing compounds (1h, 1i and 1j) showed higher activity than chloro (1c, 1d), or bromo group containing compounds (1e, 1f). Chloro and bromo derivatives also have higher activity than other rested compounds. On the basis of SAR, concluded that the activity of compounds depends on electron withdrawing nature of the substituted groups. The sequence of the activity is following NO2 > Cl > Br > OH > OCH3 > CH3. The investigation of antimicrobial (antibacterial, antifungal and antitubercular) data revealed that compounds (1c), (1d), (1e), (1f), (1h), (1i) and (1j) displayed high activity in the series, the compounds (1b), (1g) and (1m) showed moderate activity and the rest of the compounds showed less activity against all the strains compared with standard drugs. Compounds 1(a-m) were screened for their antibacterial, antifungal and anti-TB activity against selected microorganisms.35
A series of azetidinones (2a–m) (Figure 2) have been were tested for their antibacterial and antifungal activities against some selected bacteria and fungi and for their antitubercular activity against Mycobacterium tuberculosis, and their minimum inhibitory concentration (MIC) values were determined. These compounds were also exhibited anti-inflammatory activities.36 Compounds 2a–m was tested for their antibacterial, antifungal, antitubercular and anti-inflammatory activities.36
|
Compound |
Ar |
Compound |
Ar |
2a |
Phenyl |
2h |
4-Nitrophenyl |
|
2b |
4-Choloro phenyl |
2i |
3- Choloro phenyl 2- Choloro phenyl |
|
2c |
3-Choloro phenyl |
2j |
4-Methoxyphenyl |
|
2d |
2-Choloro phenyl |
2k |
4-Methylphenyl |
|
2e |
4-Bromo phenyl |
2l |
4-Hydroxyphenyl |
|
2f |
3- Bromo phenyl 2- Bromo phenyl |
2m |
||
2g |
|
|
|
Figure 2 A series of azetidinones (2a–m) have been were tested for their antibacterial and antifungal activities against some selected bacteria and fungi.
The antibacterial, antifungal and antitubercular activities of compounds 2a–m were assayed in vitro against selected bacteria: Bacillus subtilis, Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae, and fungi: Aspergillus niger, A. flavus, Candida albicans and Fusarium oxysporum, and Mtb (H37Rv) strain. The results of the antimicrobial screening data revealed that all the compounds 2a–m showed activity against the selected microorganisms. The nitro group-containing compounds (2h, 2i and 2j) showed higher activity than the chloro (2c and 2d), or bromo group containing compounds (2e and 2f). The chloro and bromo derivatives also had a higher activity than the other rested compounds. It can be concluded that the activity of the compounds depends on electron withdrawing nature of the substituent groups. The sequence of the activity is the following: NO2>Cl>Br>OH>>OCH3>CH3. The investigation of antimicrobial (antibacterial, antifungal and anti-TB) data revealed that the compounds 2c, 2d, 2e, 2f, 2h, 2i and 2j displayed high activity, the compounds 2b, 2g and 2m showed moderate activity and the other compounds showed low activity against all the strains compared with the standard drugs. In the anti-inflammatory activity test, compounds 2c, 2d, 2e, 2f, 2h, 2i and 2j showed high activity while the other compounds displayed moderate to low activity.36
A series of azetidines 3(a-j) (Figure 3) containing compounds 3(a-j) were screened for their antibacterial, antifungal and antitubercular activities in vitro against selected bacteria, B. subtilis, E. coli, S. aureus, and fungi A. niger, A. flavus, C. albicans and Mtb H37Rv strain respectively. Streptomycin and griseofulvin used as standard for antibacterial and antifungal activities respectively and isoniazid and rifampicin for anti-TB activity.
|
Compound |
X |
Compound |
X |
3a |
Phenyl |
3f |
3-bromophenyl |
|
3b |
4-chlorophenyl |
3g |
2-bromophenyl |
|
3c |
3-chlorophenyl |
3h |
4-nitrophenyl) |
|
3d |
2-chlorophenyl |
3i |
3-nitrophenyl |
|
3e |
4-bromophenyl |
3j |
2-nitrophenyl |
Figure 3 A series of azetidines 3(a-j) containing compounds 3(a-j) were screened for their antibacterial, antifungal and antitubercular activities.
The investigation of antimicrobial (antibacterial, antifungal and anti-TB) of the compounds (3c), (3d), (3e), (3f), (3h), (3i) and (3j) displayed high activity in the series, the compounds (3b) and (3g) showed moderate activity and compound 3a showed less activity against all the strains compared with standard drugs.37
A series of azetidine derivatives (4a-f) (Figure 4) were tested for their anti-bacterial activity against Staphylococcus aureus and Echerichia coli, Antifungal activity against C Albicans and anti-tubercular activity against M. tuberculosis and exhibited significant activity against bacterial, fungal and mycobacterium strains.
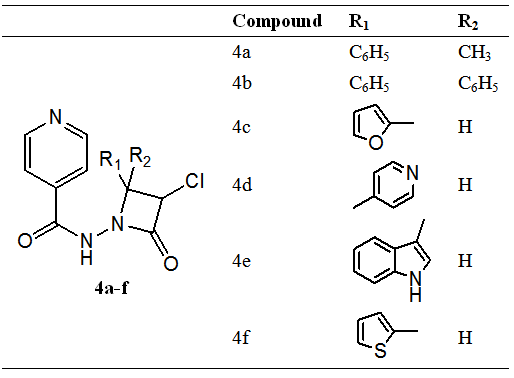
Antimicrobial screening data of compounds showed good to moderate activity, against bacterial, fungal and M. tuberculosis strain, as compared to reference drug. Compound 4a showed moderate activity against all strains. Compound 4b showed good activity against E Coli while showed moderate activity against S. aureus, C. Albicans and M. tuberculosis. Compound 4c and 4f showed good activity against all strains. Compound 4d and 4e were showed excellent antibacterial, antifungal and anti-TB activity against tested strains. The antimicrobial activity is due the presence of β-Lactam ring and increased by the addition phenyl moiety/heterocyclic compounds at 4 position of β-Lactam ring. Amongst these 4e showed the highest activity against M. tuberculosis as compare to other compounds, is due to the presence the indole moiety at the 4 position of azetidine ring.38
The 4-oxo-azetidines were also tested for their antioxidant activity. The activity of all compounds was identified by using nitric oxide and superoxide radical scavenging methods against alkaline dimethyl sulfoxide (DMSO). The derivatives (4a, 4c) with the chlorine substituent either at ortho or para on phenyl ring exhibited maximum activity in both methods. The least activity is shown by the compound (4g) having ortho nitro group on the benzene ring (Figure 5).
|
Compounds |
R= |
4a |
p-chloro |
|
4b |
m-bromo |
|
4c |
o-chloro |
|
4d |
o-methoxy |
|
4e |
o,p-dimethoxy |
|
4f |
3’,4’,5’-trimethoxy |
|
4g |
o-nitro |
Figure 5 4-oxo-azetidines were also tested for their antioxidant activity.
All the 4-oxo-azetidine derivatives (4a-4g) (Figure 5) were tested for their in vitro free radical scavenging Nitric oxide (NO) and scavenging of superoxide radical with the alkaline DMSO method. The nitric oxide assay is widely used to evaluate the free radical scavenging effectiveness of various antioxidant substances. The scavenging ability of the synthesized compounds was compared with ascorbic acid as a standard. Compounds 4c and 4e produced better scavenging ability. Compounds 4a, 4b, 4d and 4e showed moderate radical scavenging activity and 4g compound showed least activity when compared to the standard. Even though superoxide radical is a weak oxidant, it gives rise to the generation of powerful and dangerous hydroxyl radical along with single oxygen, both of which lead to oxidative stress. The compound 4c showed better scavenging activity whereas 4a, 4b and 4f exhibited moderate activity. Least activity is identified for the compounds 4d, 4e and 4g. 4-oxo-azetidines exhibited significant to moderate activity when compared with standard ascorbic acid. Strong antioxidant activity was observed for 4c in both methods.39
The antimicrobial and antitubercular activity of the synthesized compounds bearing an azetidinone moiety revealed that all the tested compounds showed antibacterial, antifungal and antitubercular activities against the selected microbial strains. Some of the compounds displayed promising activities and are of interest for further transformations towards more potent derivatives. These compounds posses good anti-bacterial, antifungal and anti-TB activities. Furthermore, the development of new azetidine derivatives which are highly desirable.
Author contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
There is no conflict of interest.

©2018 Asif. This is an open access article distributed under the terms of the, which permits unrestricted use, distribution, and build upon your work non-commercially.